Prebiotic condensation through wet-dry cycling regulated by deliquescence
- PMID: 31586058
- PMCID: PMC6778215
- DOI: 10.1038/s41467-019-11834-1
Prebiotic condensation through wet-dry cycling regulated by deliquescence
Abstract
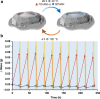
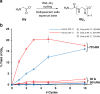
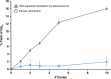
Wet-dry cycling is widely regarded as a means of driving condensation reactions under prebiotic conditions to generate mixtures of prospective biopolymers. A criticism of this model is its reliance on unpredictable rehydration events, like rainstorms. Here, we report the ability of deliquescent minerals to mediate the oligomerization of glycine during iterative wet-dry cycles. The reaction mixtures evaporate to dryness at high temperatures and spontaneously reacquire water vapor to form aqueous solutions at low temperatures. Deliquescent mixtures can foster yields of oligomerization over ten-fold higher than non-deliquescent controls. The deliquescent mixtures tightly regulate their moisture content, which is crucial, as too little water precludes dissolution of the reactants while too much water favors hydrolysis over condensation. The model also suggests a potential reason why life evolved to favor the enrichment of potassium: so living systems could acquire and retain sufficient water to serve as a solvent for biochemical reactions.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

