Synthesis of magnesium-nitrogen salts of polynitrogen anions
- PMID: 31586062
- PMCID: PMC6778147
- DOI: 10.1038/s41467-019-12530-w
Synthesis of magnesium-nitrogen salts of polynitrogen anions
Abstract
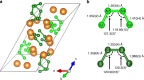
The synthesis of polynitrogen compounds is of fundamental importance due to their potential as environmentally-friendly high energy density materials. Attesting to the intrinsic difficulties related to their formation, only three polynitrogen ions, bulk stabilized as salts, are known. Here, magnesium and molecular nitrogen are compressed to about 50 GPa and laser-heated, producing two chemically simple salts of polynitrogen anions, MgN4 and Mg2N4. Single-crystal X-ray diffraction reveals infinite anionic polythiazyl-like 1D N-N chains in the crystal structure of MgN4 and cis-tetranitrogen N44- units in the two isosymmetric polymorphs of Mg2N4. The cis-tetranitrogen units are found to be recoverable at atmospheric pressure. Our results respond to the quest for polynitrogen entities stable at ambient conditions, reveal the potential of employing high pressures in their synthesis and enrich the nitrogen chemistry through the discovery of other nitrogen species, which provides further possibilities to design improved polynitrogen arrangements.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Klapötke, T. M. New Nitrogen-Rich High Explosives. in High Energy Density Materials 85–121 (Springer, 2007).
Publication types
LinkOut - more resources
Full Text Sources

