Transcriptomic profiling of the myeloma bone-lining niche reveals BMP signalling inhibition to improve bone disease
- PMID: 31586071
- PMCID: PMC6778199
- DOI: 10.1038/s41467-019-12296-1
Transcriptomic profiling of the myeloma bone-lining niche reveals BMP signalling inhibition to improve bone disease
Abstract
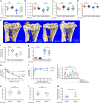
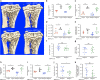
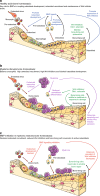
Multiple myeloma is an incurable, bone marrow-dwelling malignancy that disrupts bone homeostasis causing skeletal damage and pain. Mechanisms underlying myeloma-induced bone destruction are poorly understood and current therapies do not restore lost bone mass. Using transcriptomic profiling of isolated bone lining cell subtypes from a murine myeloma model, we find that bone morphogenetic protein (BMP) signalling is upregulated in stromal progenitor cells. BMP signalling has not previously been reported to be dysregulated in myeloma bone disease. Inhibition of BMP signalling in vivo using either a small molecule BMP receptor antagonist or a solubilized BMPR1a-FC receptor ligand trap prevents trabecular and cortical bone volume loss caused by myeloma, without increasing tumour burden. BMP inhibition directly reduces osteoclastogenesis, increases osteoblasts and bone formation, and suppresses bone marrow sclerostin levels. In summary we describe a novel role for the BMP pathway in myeloma-induced bone disease that can be therapeutically targeted.
Conflict of interest statement
H.D. is on the advisory team of Kymab Ltd. Since completing this work S.G’s research fellowship (University of Oxford) is funded by Celgene. K.R. has research grants from Celgene, Janssen, Amgen and Takeda. K.R. has received honoraria from or is on the advisory board of Celgene, Janssen, Takeda, Amgen, Abbvie and Sanofi. The other authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- G84/6443/MRC_/Medical Research Council/United Kingdom
- MC_UU_00008/10/MRC_/Medical Research Council/United Kingdom
- MC_UU_00016/15/MRC_/Medical Research Council/United Kingdom
- MC_UU_12009/16/MRC_/Medical Research Council/United Kingdom
- MC_PC_15065/MRC_/Medical Research Council/United Kingdom
- NC/M000133/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
- 27723/CRUK_/Cancer Research UK/United Kingdom
- MR/L006340/1/MRC_/Medical Research Council/United Kingdom
- R01 GM121499/GM/NIGMS NIH HHS/United States
- 27125/CRUK_/Cancer Research UK/United Kingdom
- 20631/VAC_/Versus Arthritis/United Kingdom
- 26988/CRUK_/Cancer Research UK/United Kingdom
- MC_UU_12010/10/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

