Different vascular healing process between bioabsorbable polymer-coated everolimus-eluting stents versus bioresorbable vascular scaffolds via optical coherence tomography and coronary angioscopy (the ENHANCE study: ENdothelial Healing Assessment with Novel Coronary tEchnology)
- PMID: 31587103
- PMCID: PMC7085473
- DOI: 10.1007/s00380-019-01516-9
Different vascular healing process between bioabsorbable polymer-coated everolimus-eluting stents versus bioresorbable vascular scaffolds via optical coherence tomography and coronary angioscopy (the ENHANCE study: ENdothelial Healing Assessment with Novel Coronary tEchnology)
Abstract
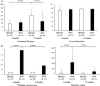
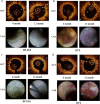
Recent clinical trials have raised concerns about the safety and efficacy of ABSORB™ bioresorbable vascular scaffolds (BVS). The difference in the vascular healing process between SYNERGY™ bioabsorbable polymer-coated everolimus-eluting stents (BP-EES) and BVS remains unclear. The aim of the ENHANCE study was to compare vascular healing on BP-EES versus BVS by optical coherence tomography (OCT) and coronary angioscopy (CAS) at 4- and 12-month follow-ups. This is a prospective, non-randomized, single center clinical trial. Thirteen eligible patients with multivessel disease were enrolled. BP-EES and BVS were simultaneously implanted in the same patients, but in different coronary vessels. Imaging follow-up with both OCT and CAS was completed in 11 patients at 12 months. Neointimal coverage rates were similar between the two groups based on OCT measurements. The neointimal thickness of BP-EES was significantly thicker at the 12th month than at the 4th month, whereas the neointimal thickness of BVS did not change between the measurements taken at the 4th and 12th month. Existence of intra-stent thrombus was significantly higher in the BVS group, compared to the BP-EES group. On the other hand, CAS revealed that red-thrombi and yellow-plaque were more frequently observed in BVS at 4 months and up to 12-month follow-ups than in BP-EES. These findings suggested that the evidence of instability remained up to 12 months in the vascular healing with BVS, compared to that with BP-EES. Vascular healing of the stented wall was recognized at the very early phase after BP-EES implantation. However, vascular healing with BVS was still incomplete after 12 months.
Keywords: Bioabsorbable polymer-coated everolimus-eluting stents; Bioresorbable vascular scaffolds; Coronary angioscopy; Optical coherence tomography; Vascular healing.
Conflict of interest statement
All authors declare that they have no conflict of interest.
Figures
Similar articles
-
Vascular response to everolimus- and biolimus-eluting coronary stents versus everolimus-eluting bioresorbable scaffolds--an optical coherence tomography substudy of the EVERBIO II trial.Swiss Med Wkly. 2016 Jan 14;146:w14274. doi: 10.4414/smw.2016.14274. eCollection 2016. Swiss Med Wkly. 2016. PMID: 26766027 Clinical Trial.
-
Comparison of neointimal coverage between durable-polymer everolimus-eluting stents and bioresorbable-polymer everolimus-eluting stents 1 year after implantation using high-resolution coronary angioscopy.Catheter Cardiovasc Interv. 2019 Aug 1;94(2):204-209. doi: 10.1002/ccd.28095. Epub 2019 Feb 9. Catheter Cardiovasc Interv. 2019. PMID: 30737973
-
Two-year clinical, angiographic, and serial optical coherence tomographic follow-up after implantation of an everolimus-eluting bioresorbable scaffold and an everolimus-eluting metallic stent: insights from the randomised ABSORB Japan trial.EuroIntervention. 2016 Oct 20;12(9):1090-1101. doi: 10.4244/EIJY16M09_01. EuroIntervention. 2016. PMID: 27597270 Clinical Trial.
-
Everolimus-Eluting Bioresorbable Scaffolds Versus Everolimus-Eluting Metallic Stents.J Am Coll Cardiol. 2017 Jun 27;69(25):3055-3066. doi: 10.1016/j.jacc.2017.04.011. Epub 2017 Apr 12. J Am Coll Cardiol. 2017. PMID: 28412389 Review.
-
Everolimus-eluting bioresorbable vascular scaffolds versus everolimus-eluting metallic stents: a meta-analysis of randomised controlled trials.Lancet. 2016 Feb 6;387(10018):537-544. doi: 10.1016/S0140-6736(15)00979-4. Epub 2015 Nov 17. Lancet. 2016. PMID: 26597771 Review.
Cited by
-
Recent advances in Fe-based bioresorbable stents: Materials design and biosafety.Bioact Mater. 2023 Aug 26;31:333-354. doi: 10.1016/j.bioactmat.2023.07.024. eCollection 2024 Jan. Bioact Mater. 2023. PMID: 37663617 Free PMC article. Review.
References
-
- Virmani R, Guagliumi G, Farb A, Musumeci G, Grieco N, Motta T, Mihalcsik L, Tespili M, Valsecchi O, Kolodgie FD. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation. 2004;109:701–705. doi: 10.1161/01.CIR.0000116202.41966.D4. - DOI - PubMed
-
- Tellez A, Seifert PS, Donskoy E, Sushkova N, Pennington DE, Milewski K, Krueger CG, Kaluza GL, Eppjhimer MJ, Huibregtse BA, Dawkins KD, Granada JF. Experimental evaluation of efficacy and healing response of everolimus-eluting stents in the familial, hypercholesterolemic swine model: a comparative study of bioabsorbable versus durable polymer stent platforms. Coron Artery Dis. 2014;25:198–207. doi: 10.1097/MCA.0000000000000134. - DOI - PubMed
-
- Meredith IT, Verheye S, Dubois CL, Dens J, Fajadet J, Carrie D, Walsh S, Oldroyd KG, Varenne O, El-Jack S, Moreno R, Joshi AA, Allocco DJ, Dawkins KD. Primary endpoint results of the EVOLVE trial: a randomized evaluation of a novel bioabsorbable polymer-, neointimal coated, everolimus-esluting coronary stent. J Am Coll Cardiol. 2012;59:1362–1370. doi: 10.1016/j.jacc.2011.12.016. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

