Synaptic GABAergic transmission in the central amygdala (CeA) of rats depends on slice preparation and recording conditions
- PMID: 31587506
- PMCID: PMC6778595
- DOI: 10.14814/phy2.14245
Synaptic GABAergic transmission in the central amygdala (CeA) of rats depends on slice preparation and recording conditions
Abstract
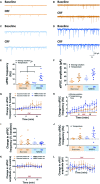
The central nucleus of the amygdala (CeA) is a primarily GABAergic brain region implicated in stress and addictive disorders. Using in vitro slice electrophysiology, many studies measure GABAergic neurotransmission to evaluate the impact of experimental manipulations on inhibitory tone in the CeA, as a measure of alterations in CeA activity and function. In a recent study, we reported spontaneous inhibitory postsynaptic current (sIPSC) frequencies higher than those typically reported in CeA neurons in the literature, despite utilizing similar recording protocols and internal recording solutions. The purpose of this study was to systematically evaluate two common methods of slice preparation, an NMDG-based aCSF perfusion method and an ice-cold sucrose solution, as well as the use of an in-line heater to control recording temperature, on measures of intrinsic excitability and spontaneous inhibitory neurotransmission in CeA neurons. We report that both slice preparation and recording conditions significantly impact spontaneous GABAergic transmission in CeA neurons, and that recording temperature, but not slicing solution, alters measures of intrinsic excitability in CeA neurons. Bath application of corticotropin-releasing factor (CRF) increased sIPSC frequency under all conditions, but the magnitude of this effect was significantly different across recording conditions that elicited different baseline GABAergic transmission. Furthermore, CRF effects on synaptic transmission differed according to data reporting methods (i.e., raw vs. normalized data), which is important to consider in relation to baseline synaptic transmission values. These studies highlight the impact of experimental conditions and data reporting methods on neuronal excitability and synaptic transmission in the CeA.
Keywords: CRF; NMDG; central amygdala; electrophysiology; neurotransmission.
© 2019 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
NWG owns shares in Glauser Life Sciences, Inc., a start‐up company with interest in development of therapeutics for treatment of mental illness. All other authors declare no competing financial interests.
Figures
References
-
- Aghajanian, G. K. , and Rasmussen K.. 1989. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse 3:331–338. - PubMed
-
- Baginskas, A. , Palani D., Chiu K., and Raastad M.. 2009. The H‐current secures action potential transmission at high frequencies in rat cerebellar parallel fibers. Eur. J. Neurosci. 29:87–96. - PubMed
-
- Brahma, B. , Forman R. E., Stewart E. E., Nicholson C., and Rice M. E.. 2000. Ascorbate inhibits edema in brain slices. J. Neurochem. 74:1263–1270. - PubMed
-
- Cao, X.‐J. , and Oertel D.. 2005. Temperature Affects voltage‐sensitive conductances differentially in octopus cells of the mammalian cochlear nucleus. J. Neurophysiol. 94:821–832. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

