Divergent paths to seizure-like events
- PMID: 31587522
- PMCID: PMC6778598
- DOI: 10.14814/phy2.14226
Divergent paths to seizure-like events
Abstract
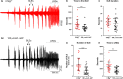
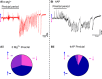
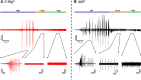
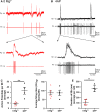
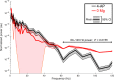
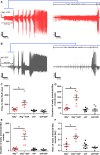
Much debate exists about how the brain transitions into an epileptic seizure. One source of confusion is that there are likely to be critical differences between experimental seizure models. To address this, we have compared the evolving activity patterns in two widely used in vitro models of epileptic discharges. Brain slices from young adult mice were prepared in the same way and bathed either in 0 Mg2+ or 100 µmol/L 4AP artificial cerebrospinal fluid. We have found that while local field potential recordings of epileptiform discharges in the two models appear broadly similar, patch-clamp analysis reveals an important difference in the relative degree of glutamatergic involvement. 4AP affects parvalbumin-expressing interneurons more than other cortical populations, destabilizing their resting state and inducing spontaneous bursting behavior. Consequently, the most prominent pattern of transient discharge ("interictal event") in this model is almost purely GABAergic, although the transition to seizure-like events (SLEs) involves pyramidal recruitment. In contrast, interictal discharges in 0 Mg2+ are only maintained by a very large glutamatergic component that also involves transient discharges of the interneurons. Seizure-like events in 0 Mg2+ have significantly higher power in the high gamma frequency band (60-120Hz) than these events do in 4AP, and are greatly delayed in onset by diazepam, unlike 4AP events. We, therefore, conclude that the 0 Mg2+ and 4AP models display fundamentally different levels of glutamatergic drive, demonstrating how ostensibly similar pathological discharges can arise from different sources. We contend that similar interpretative issues will also be relevant to clinical practice.
Keywords: Epilepsy; ictal events; interictal events; interneurons.
© 2019 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Anderson, W. W. , Lewis D. V., Swartzwelder H. S., and Wilson W. A.. 1986. Magnesium‐free medium activates seizure‐like events in the rat hippocampal slice. Brain Res. 398:215–219. - PubMed
-
- Avoli, M. , D'Antuono M., Louvel J., Kohling R., Biagini G., Pumain R., et al. 2002. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog. Neurogibol. 68:167–207. - PubMed
-
- Bordey, A. , and Sontheimer H.. 1999. Differential inhibition of glial K(+) currents by 4‐AP. J. Neurophysiol. 82:3476–3487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

