Functionally Distinct Gamma Range Activity Revealed by Stimulus Tuning in Human Visual Cortex
- PMID: 31588003
- PMCID: PMC6810857
- DOI: 10.1016/j.cub.2019.08.004
Functionally Distinct Gamma Range Activity Revealed by Stimulus Tuning in Human Visual Cortex
Abstract
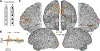
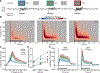
Neocortical gamma activity has long been hypothesized as a mechanism for synchronizing brain regions to support visual perception and cognition more broadly. Although early studies focused on narrowband gamma oscillations (∼20-60 Hz), recent work has emphasized a more broadband "high-gamma" response (∼70-150+ Hz). These responses are often conceptually or analytically treated as synonymous markers of gamma activity. Using high-density intracranial recordings from the human visual cortex, we challenge this view by showing distinct spectral, temporal, and functional properties of narrow and broadband gamma. Across four experiments, narrowband gamma was strongly selective for gratings and long-wavelength colors, displaying a delayed response onset, sustained temporal profile, and contrast-dependent peak frequency. In addition, induced narrowband gamma oscillations lacked phase consistency across stimulus repetitions and displayed highly focal inter-site synchronization. In contrast, broadband gamma was consistently observed for all presented stimuli, displaying a rapid response onset, transient temporal profile, and invariant spectral properties. We exploited stimulus tuning to highlight the functional dissociation of these distinct signals, reconciling prior inconsistencies across species and stimuli regarding the ubiquity of visual gamma oscillations during natural vision. The occurrence of visual narrowband gamma oscillations, unlike broadband high gamma, appears contingent on specific structural and chromatic stimulus attributes intersecting with the receptive field. Together, these findings have important implications for the study, analysis, and functional interpretation of neocortical gamma-range activity.
Keywords: broadband gamma; color vision; electrocorticography; gamma oscillations; high-frequency activity; human vision; narrowband gamma; natural vision; neural synchronization; visual cortex.
Copyright © 2019 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures





References
-
- Fries P (2005). A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn. Sci 9, 474–480. - PubMed
-
- Fries P (2009). Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu. Rev. Neurosci 32, 209–224. - PubMed
-
- Singer W (1999). Neuronal synchrony: a versatile code for the definition of relations? Neuron 24, 49–65, 111–125. - PubMed
-
- Gray CM (1999). The temporal correlation hypothesis of visual feature integration: still alive and well. Neuron 24, 31–47, 111–125. - PubMed
-
- Singer W, and Gray CM (1995). Visual feature integration and the temporal correlation hypothesis. Annu. Rev. Neurosci 18, 555–586. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

