Statin Intolerance Clinical Guide 2018
- PMID: 31588101
- PMCID: PMC7192817
- DOI: 10.5551/jat.50948
Statin Intolerance Clinical Guide 2018
Keywords: Liver injury; Myopathy; Renal dysfunction; Rhabdomyolysis; Statin intolerance.
Conflict of interest statement
In accordance with the “COI Management Guidelines for Clinical Research” established by the Japan Association of Medical Sciences' COI committee, a conflict of interest (COI) statement has been obtained form each member of the committee involved in drafting the Statin Intolerance Clinical Guide 2018. The names of the enterprises disclosed in the COI statement are provided below. The applicable period is January 01, 2015, to December 31, 2017.
Abbvie, Astellas Amgen BioPharma, Astellas Pharma, Bayer, Boehringer Ingelheim Japan, Bristol- Myers Squibb, Central Medical, Daiichi Sankyo, Dai- Nihon Insatsu Kenko Hoken Kumiai, East Japan Institute of Technology, Eisai, Fujifilm, Gilead Sciences, Japan Data Center, Kowa, Kusatsu City Hall, Kyowa Medics, Medical Review, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, MSD, Municipal Kaizuka Hospital, Novartis Pharma, Nakamura Hospital, Novo Nordisk, Ono Pharmaceutical, Otsuka Pharmaceutical, Pfizer Japan, Rinku General Medical Center, Rohto Pharmaceutical, Sanofi, Sanwa Kagaku Kenkyusho, Skylight Biotech, St. Jude Medical, Sumitomo Dainippon Pharma, Takeda Pharmaceuctical, the City of Izumisano,.
Figures
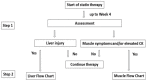
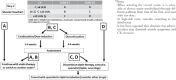
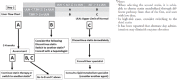


References
-
- Endo A. The discovery and development of HMG-CoA reductase inhibitors. J Lipid Res, 1992; 33: 1569-1582 - PubMed
-
- Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, Egusa G, Hiro T, Hirobe K, Iida M, Kihara S, Kinoshita M, Maruyama C, Ohta T, Okamura T, Yamashita S, Yokode M, Yokote K. Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan — 2012 version. J Atheroscler Thromb, 2013; 20: 517-523 - PubMed
-
- Fitchett DH, Hegele RA, Verma S. Cardiology patient page. Statin intolerance. Circulation, 2015; 31: 131 - PubMed
-
- Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, Roden M, Stein E, Tokgözoğlu L, Nordestgaard BG, Bruckert E, De Backer G, Krauss RM, Laufs U, Santos RD, Hegele RA, Hovingh GK, Leiter LA, Mach F, Marz W, Newman CB, Wiklund O, Jacobson TA, Catapano AL, Chapman MJ, Ginsberg HN: European Atherosclerosis Society Consensus Panel. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J, 2015; 36: 1012-1022 - PMC - PubMed
-
- Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM, IMPROVE-IT Investigators Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med, 2015; 372: 2387-2397 - PubMed

