Intracellular MicroRNA imaging using telomerase-catalyzed FRET ratioflares with signal amplification
- PMID: 31588279
- PMCID: PMC6677110
- DOI: 10.1039/c9sc02301a
Intracellular MicroRNA imaging using telomerase-catalyzed FRET ratioflares with signal amplification
Abstract
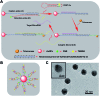
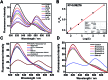
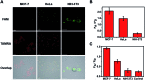
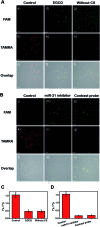
Intracellular microRNA (miRNA) detection has attracted increasing attention, resulting in significant achievements. However, the development of an available tool that possesses a satisfactory signal-to-background ratio and high sensitivity for miRNA detection remains challenging. Herein, a class of telomerase-catalyzed FRET (fluorescence resonance energy transfer) ratioflares has been developed for the accurate sensing of low-abundance cancer-related miRNA both in a fluorescence assay and living cell imaging with signal amplification capacity. In this work, endogenous telomerase is led in a miRNA test system with signal amplification for the first time, wherein telomerase extends hexamer telomeric repeats (TTAGGG) using the 3' end of the capture probe as the primer. The synergetic work of telomerase and the catalyst strand (CS) makes the target miRNA circulate in the system, resulting in high sensitivity and an enhanced FRET signal an improved detection limit of 2.27 × 10-15 M. Meanwhile, telomerase-catalyzed FRET ratioflares allow the difference between cancer cells and normal cells to be increased reliably. Furthermore, low false positive signals resulting from chemical interference and minimized system fluctuations are achieved through ratiometric measurements.
This journal is © The Royal Society of Chemistry 2019.
Figures

References
-
- Almeida M. I., Reis R. M., Calin G. A. Mutat. Res., Fundam. Mol. Mech. Mutagen. 2011;717:1. - PubMed
-
- Lu J., Getz G., Miska E. A., Alvarez-Saavedra E., Lamb J., Peck D., Sweet-Cordero A., Ebert B. L., Mak R. H., Ferrando A. A., Downing J. R., Jacks T., Horvitz H. R., Golub T. R. Nature. 2005;435:834. - PubMed
-
- Bartel D. P. Cell. 2004;116:281. - PubMed
LinkOut - more resources
Full Text Sources

