Palladium-catalyzed enantioselective alkenylation of alkenylbenzene derivatives
- PMID: 31588293
- PMCID: PMC6685350
- DOI: 10.1039/c9sc02380a
Palladium-catalyzed enantioselective alkenylation of alkenylbenzene derivatives
Abstract
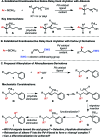
A regioselective and enantioselective palladium-catalyzed relay Heck alkenylation of alkenylbenzene derivatives to construct remote stereocenters is disclosed. Various β-substituted styrenes were readily obtained in moderate yields with good to excellent levels of enantioselectivity. This strategy provides rapid access to enantioenriched δ, ε, ζ, and η-alkenyl aryl compounds from simple starting materials. Mechanistic studies suggest that termination of the relay reaction is controlled by affinity of the arene for the Pd complex during migration.
This journal is © The Royal Society of Chemistry 2019.
Figures
References
-
-
For selected reviews, see:
- Uma R., Crévisy C., Grée R. Chem. Rev. 2003;103:27. - PubMed
- Kuznik N., Krompiec S. Coord. Chem. Rev. 2007;251:222.
- Krompiec S., Krompiec M., Penczek R., Ignasiak H. Coord. Chem. Rev. 2008;252:1819.
- Larionov E., Li H., Mazet C. Chem. Commun. 2014;50:9816. - PubMed
- Hassam M., Taher A., Arnott G. E., Green I. R., van Otterlo W. A. L. Chem. Rev. 2015;115:5462. - PubMed
- Vilches-Herrera M., Domke L., Börner A. ACS Catal. 2014;4:1706.
- Vasseur A., Bruffaerts J., Marek I. Nat. Chem. 2016;8:209. - PubMed
- Sommer H., Juliá-Hernández F., Martin R., Marek I. ACS Cent. Sci. 2018;4:153. - PMC - PubMed
-
-
-
Selected examples: for Rh-catalyzed chain walking, see ; for Pd-catalyzed chain walking, see ; for Co-catalyzed chain walking, see ; for Ni-catalyzed chain walking, see
- Yoshida K., Hayashi T. J. Am. Chem. Soc. 2003;125:2872. - PubMed
- Borah A. J., Shi Z. J. Am. Chem. Soc. 2018;140:6062. - PubMed
- Oliveira C. C., Angnes R. A., Correia C. R. D. J. Org. Chem. 2013;78:4373. - PubMed
- Larionov E., Lin L., Guénée L., Mazet C. J. Am. Chem. Soc. 2014;136:16882. - PubMed
- Hamasaki T., Aoyama Y., Kawasaki J., Kakiuchi F., Kochi T. J. Am. Chem. Soc. 2015;137:16163. - PubMed
- Singh S., Bruffaerts J., Vasseur A., Marek I. Nat. Commun. 2017;8:14200. - PMC - PubMed
- Kohler D. G., Gockel S. N., Kennemur J. L., Waller P. J., Hull K. L. Nat. Chem. 2018;10:333. - PMC - PubMed
- Obligacion J. V., Chirik P. J. J. Am. Chem. Soc. 2013;135:19107. - PubMed
- Lee W.-C., Wang C.-H., Lin Y.-H., Shih W.-C., Ong T.-G. Org. Lett. 2013;15:5358. - PubMed
- He Y., Cai Y., Zhu S. J. Am. Chem. Soc. 2017;139:1061. - PubMed
- Juliá-Hernández F., Moragas T., Cornella J., Martin R. Nature. 2017;545:84. - PubMed
- Peng L., Li Y., Li Y., Wang W., Pang H., Yin G. ACS Catal. 2018;8:310.
- Wang Z., Yin H., Fu G. C. Nature. 2018;563:379. - PMC - PubMed
-
-
-
SelectedSelected examples: examples:
- Mei T.-S., Werner E. W., Burckle A. J., Sigman M. S. J. Am. Chem. Soc. 2013;135:6830. - PMC - PubMed
- Mei T.-S., Patel H. H., Sigman M. S. Nature. 2014;508:340. - PMC - PubMed
- Zhang C., Santiago C. B., Crawford J. M., Sigman M. S. J. Am. Chem. Soc. 2015;137:15668. - PMC - PubMed
- Chen Z.-M., Hilton M. J., Sigman M. S. J. Am. Chem. Soc. 2016;138:11461. - PMC - PubMed
- Race N. J., Schwalm C. S., Nakamuro T., Sigman M. S. J. Am. Chem. Soc. 2016;138:15881. - PMC - PubMed
- Chen Z.-M., Nervig C. S., Deluca R. J., Sigman M. S. Angew. Chem., Int. Ed. 2017;56:6651. - PMC - PubMed
- Yuan Q., Sigman M. S. J. Am. Chem. Soc. 2018;140:6527. - PMC - PubMed
-
Grants and funding
LinkOut - more resources
Full Text Sources