Substituent-controlled, mild oxidative fluorination of iodoarenes: synthesis and structural study of aryl I(iii)- and I(v)-fluorides
- PMID: 31588294
- PMCID: PMC6685355
- DOI: 10.1039/c9sc02162k
Substituent-controlled, mild oxidative fluorination of iodoarenes: synthesis and structural study of aryl I(iii)- and I(v)-fluorides
Abstract
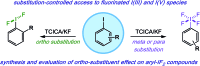
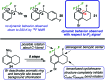
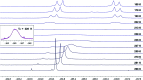
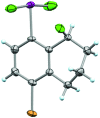
We report a mild approach to the synthesis of difluoro(aryl)-λ3-iodanes (aryl-IF2 compounds) and tetrafluoro(aryl)-λ5-iodanes (aryl-IF4 compounds) using trichloroisocyanuric acid (TCICA) and potassium fluoride (KF). Under these reaction conditions, selective access to either the I(iii)- or I(v)-derivatives is predictable based solely on the substitution pattern of the iodoarene starting material. Moreover, the discovery of this TCICA/KF approach prompted detailed dynamic NMR, kinetic, computational, and crystallographic studies on the relationship between the IF2 group and the ortho-substituents on carefully designed probe molecules. It was during these experiments that the role of the ortho-substituent in inhibiting further oxidative fluorination of I(iii)-compounds to I(v)-compounds during the reaction with TCICA and KF was revealed. Additionally, a notable exception to this empirical trend is discussed herein.
This journal is © The Royal Society of Chemistry 2019.
Figures

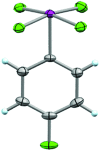

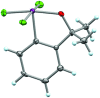
References
-
- Zhdankin V. V., Hypervalent Iodine Chemistry: Preparation, Structure and Synthetic Applications of Polyvalent Iodine Compounds, John Wiley & Sons, West Sussex, UK, 2014.
-
- Ye C., Twamley B., Shreeve J. M. Org. Lett. 2005;7:3961. - PubMed
-
- Sarie J. C., Thiehoff C., Mudd R. J., Daniliuc C. G., Kehr G., Gilmour R. J. Org. Chem. 2017;82:11792. - PubMed
-
-
For some examples, see:
- Zupan M., Pollak A. J. Fluorine Chem. 1976;7:445.
- Ruppert I. J. Fluorine Chem. 1980;15:173.
- Naumann D., Rüther G. J. Fluorine Chem. 1980;15:213.
- Alam K., Janzen A. F. J. Fluorine Chem. 1987;36:179.
- Hara S., Yoshida M., Fukuhara T., Yoneda N. Chem. Commun. 1998:965.
- Padelidakis V., Tyrra W., Naumann D. J. Fluorine Chem. 1999;99:9.
- Frohn H. J., Bardin V. V. J. Fluorine Chem. 2005;126:1036.
- Ye C., Twamley B., Shreeve J. M. Org. Lett. 2005;7:3961. - PubMed
- Suzuki S., Kamo T., Fukushi K., Hiramatsu T., Tokunaga E., Dohi T., Kita Y., Shibata N. Chem. Sci. 2014;5:2754.
- Banik S. M., Medley J. W., Jacobsen E. N. J. Am. Chem. Soc. 2016;138:5000. - PMC - PubMed
- Haupt J. D., Berger M., Waldvogel S. R. Org. Lett. 2019;21:242. - PubMed
-

