Chromogranin A and its fragments in cardiovascular, immunometabolic, and cancer regulation
- PMID: 31588572
- PMCID: PMC6899468
- DOI: 10.1111/nyas.14249
Chromogranin A and its fragments in cardiovascular, immunometabolic, and cancer regulation
Abstract
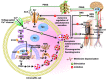
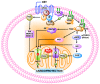
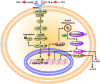
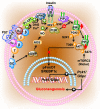
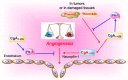
Chromogranin A (CgA)-the index member of the chromogranin/secretogranin secretory protein family-is ubiquitously distributed in endocrine, neuroendocrine, and immune cells. Elevated levels of CgA-related polypeptides, consisting of full-length molecules and fragments, are detected in the blood of patients suffering from neuroendocrine tumors, heart failure, renal failure, hypertension, rheumatoid arthritis, and inflammatory bowel disease. Full-length CgA and various CgA-derived peptides, including vasostatin-1, pancreastatin, catestatin, and serpinin, are expressed at different relative levels in normal and pathological conditions and exert diverse, and sometime opposite, biological functions. For example, CgA is overexpressed in genetic hypertension, whereas catestatin is diminished. In rodents, the administration of catestatin decreases hypertension, cardiac contractility, obesity, atherosclerosis, and inflammation, and it improves insulin sensitivity. By contrast, pancreastatin is elevated in diabetic patients, and the administration of this peptide to obese mice decreases insulin sensitivity and increases inflammation. CgA and the N-terminal fragment of vasostatin-1 can enhance the endothelial barrier function, exert antiangiogenic effects, and inhibit tumor growth in animal models, whereas CgA fragments lacking the CgA C-terminal region promote angiogenesis and tumor growth. Overall, the CgA system, consisting of full-length CgA and its fragments, is emerging as an important and complex player in cardiovascular, immunometabolic, and cancer regulation.
Keywords: cancer; cardiovascular diseases; catestatin; immunometabolism; pancreastatin; vasostatin.
© 2019 The Authors. Annals of the New York Academy of Sciences published by Wiley Periodicals, Inc. on behalf of New York Academy of Sciences.
Figures

References
-
- Blaschko, H. , Comline R.S., Schneider F.H., et al 1967. Secretion of a chromaffin granule protein, chromogranin, from the adrenal gland after splanchnic stimulation. Nature 215: 58–59. - PubMed
-
- Konecki, D.S. , Benedum U.M., Gerdes H.H., et al 1987. The primary structure of human chromogranin A and pancreastatin. J. Biol. Chem. 262: 17026–17030. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

