Stimulation of Piezo1 by mechanical signals promotes bone anabolism
- PMID: 31588901
- PMCID: PMC6779475
- DOI: 10.7554/eLife.49631
Stimulation of Piezo1 by mechanical signals promotes bone anabolism
Abstract
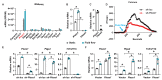
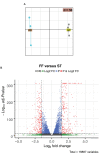
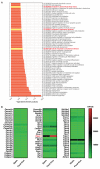
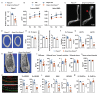
Mechanical loading, such as caused by exercise, stimulates bone formation by osteoblasts and increases bone strength, but the mechanisms are poorly understood. Osteocytes reside in bone matrix, sense changes in mechanical load, and produce signals that alter bone formation by osteoblasts. We report that the ion channel Piezo1 is required for changes in gene expression induced by fluid shear stress in cultured osteocytes and stimulation of Piezo1 by a small molecule agonist is sufficient to replicate the effects of fluid flow on osteocytes. Conditional deletion of Piezo1 in osteoblasts and osteocytes notably reduced bone mass and strength in mice. Conversely, administration of a Piezo1 agonist to adult mice increased bone mass, mimicking the effects of mechanical loading. These results demonstrate that Piezo1 is a mechanosensitive ion channel by which osteoblast lineage cells sense and respond to changes in mechanical load and identify a novel target for anabolic bone therapy.
Keywords: Piezo1; bone homeostasis; human biology; mechanosensation; medicine; mouse; osteocytes.
© 2019, Li et al.
Conflict of interest statement
XL, LH, IN, EM, MS, MA, JX No competing interests declared
Figures
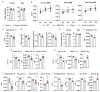
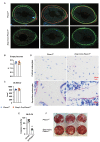
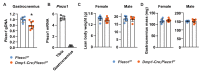
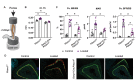



Comment in
-
Sensing the load.Elife. 2019 Oct 7;8:e50210. doi: 10.7554/eLife.50210. Elife. 2019. PMID: 31588900 Free PMC article.
References
-
- Bivi N, Condon KW, Allen MR, Farlow N, Passeri G, Brun LR, Rhee Y, Bellido T, Plotkin LI. Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. Journal of Bone and Mineral Research. 2012;27:374–389. doi: 10.1002/jbmr.548. - DOI - PMC - PubMed
-
- Choi D, Park E, Jung E, Cha B, Lee S, Yu J, Kim PM, Lee S, Hong YJ, Koh CJ, Cho CW, Wu Y, Li Jeon N, Wong AK, Shin L, Kumar SR, Bermejo-Moreno I, Srinivasan RS, Cho IT, Hong YK. Piezo1 incorporates mechanical force signals into the genetic program that governs lymphatic valve development and maintenance. JCI Insight. 2019;4:125068. doi: 10.1172/jci.insight.125068. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

