Visualizing endogenous opioid receptors in living neurons using ligand-directed chemistry
- PMID: 31589142
- PMCID: PMC6809603
- DOI: 10.7554/eLife.49319
Visualizing endogenous opioid receptors in living neurons using ligand-directed chemistry
Abstract
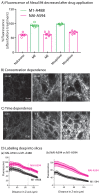
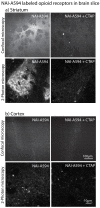
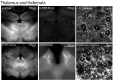
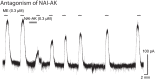
Identifying neurons that have functional opioid receptors is fundamental for the understanding of the cellular, synaptic and systems actions of opioids. Current techniques are limited to post hoc analyses of fixed tissues. Here we developed a fluorescent probe, naltrexamine-acylimidazole (NAI), to label opioid receptors based on a chemical approach termed 'traceless affinity labeling'. In this approach, a high affinity antagonist naltrexamine is used as the guide molecule for a transferring reaction of acylimidazole at the receptor. This reaction generates a fluorescent dye covalently linked to the receptor while naltrexamine is liberated and leaves the binding site. The labeling induced by this reagent allowed visualization of opioid-sensitive neurons in rat and mouse brains without loss of function of the fluorescently labeled receptors. The ability to locate endogenous receptors in living tissues will aid considerably in establishing the distribution and physiological role of opioid receptors in the CNS of wild type animals.
Keywords: endogenous; mouse; neuroscience; opioid receptors; rat; wildtype.
Conflict of interest statement
SA, AP, EP, HJ, TM, WB, KR, DF, JW No competing interests declared
Figures


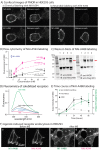
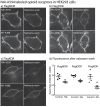
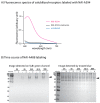
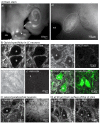




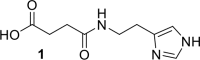
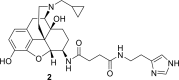
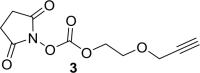
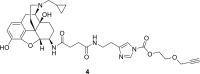
References
-
- Arttamangkul S, Ishmael JE, Murray TF, Grandy DK, DeLander GE, Kieffer BL, Aldrich JV. Synthesis and opioid activity of conformationally constrained dynorphin A analogues. 2. conformational constraint in the "address" sequence. Journal of Medicinal Chemistry. 1997;40:1211–1218. doi: 10.1021/jm960753p. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

