Differential impact of two critical respiratory centres in opioid-induced respiratory depression in awake mice
- PMID: 31589332
- PMCID: PMC6938533
- DOI: 10.1113/JP278612
Differential impact of two critical respiratory centres in opioid-induced respiratory depression in awake mice
Abstract
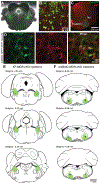
Key points: The main cause of death from opioid overdose is respiratory depression due to the activation of µ-opioid receptors (MORs). We conditionally deleted MORs from neurons in two key areas of the brainstem respiratory circuitry (the Kölliker-Fuse nucleus (KF) and pre-Bötzinger complex (preBötC)) to determine their role in opioid-induced respiratory disturbances in adult, awake mice. Deletion of MORs from KF neurons attenuated respiratory rate depression at all doses of morphine. Deletion of MORs from preBötC neurons attenuated rate depression at the low dose, but had no effect on rate following high doses of morphine. Instead, high doses of morphine increased the occurrence of apnoeas. The results indicate that opioids affect distributed key areas of the respiratory network in a dose-dependent manner and countering the respiratory effects of high dose opioids via the KF may be an effective approach to combat overdose.
Abstract: The primary cause of death from opioid overdose is respiratory failure. High doses of opioids cause severe rate depression and increased risk of fatal apnoea, which correlate with increasing irregularities in breathing pattern. µ-Opioid receptors (MORs) are widely distributed throughout the brainstem respiratory network, but the mechanisms underlying respiratory depression are poorly understood. The medullary pre-Bötzinger complex (preBötC) and the pontine Kölliker-Fuse nucleus (KF) are considered critical for inducing opioid-related respiratory disturbances. We used a conditional knockout approach to investigate the roles and relative contribution of MORs in KF and preBötC neurons in opioid-induced respiratory depression in awake adult mice. The results revealed dose-dependent and region-specific opioid effects on the control of both respiratory rate and pattern. Respiratory depression induced by an anti-nociceptive dose of morphine was significantly attenuated following deletion of MORs from either the KF or the preBötC, suggesting cumulative network effects on respiratory rate control at low opioid doses. Deletion of MORs from KF neurons also relieved rate depression at near-maximal respiratory depressant doses of morphine. Meanwhile, deletion of MORs from the preBötC had no effect on rate following administration of high doses of morphine. Instead, a severe ataxic breathing pattern emerged with many apnoeas. We conclude that opioids affect distributed areas of the respiratory network and opioid-induced respiratory depression cannot be attributed to only one area in isolation. However, countering the effects of near maximal respiratory depressant doses of opioids in the KF may be a powerful approach to combat opioid overdose.
Keywords: Kolliker-Fuse nucleus; MOR conditional knockout; morphine; mu-opioid receptors; pre-Bötzinger complex; respiratory depression.
© 2019 The Authors. The Journal of Physiology © 2019 The Physiological Society.
Conflict of interest statement
Figures





Comment in
-
Finding inspiration in opioid-induced respiratory depression.J Physiol. 2020 Jan;598(1):3. doi: 10.1113/JP279083. Epub 2019 Dec 21. J Physiol. 2020. PMID: 31675443 Free PMC article. No abstract available.
References
-
- Bouillon T, Bruhn J, Roepcke H & Hoeft A (2003). Opioid-induced respiratory depression is associated with increased tidal volume variability. Eur J Anaesthesiol 20, 127–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

