Single Electron Transfer to Diazomethane-Borane Adducts Prompts C-H Bond Activations
- PMID: 31589360
- PMCID: PMC6972512
- DOI: 10.1002/anie.201912338
Single Electron Transfer to Diazomethane-Borane Adducts Prompts C-H Bond Activations
Abstract
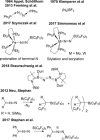
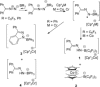
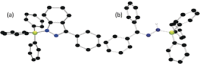
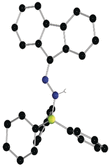
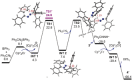
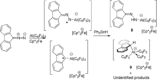
While (Ph2 CN2 )B(C6 F5 )3 is unstable, single electron transfer from Cp*2 Co affords the isolation of stable products [Cp*2 Co][Ph2 CNNHB(C6 F5 )3 ] 1 and [Cp*Co(C5 Me4 CH2 B(C6 F5 )3 )] 2. The analogous combination of Ph2 CN2 and BPh3 showed no evidence of adduct formation and yet single electron transfer from Cp*2 Cr affords the species [Cp*2 Cr][PhC(C6 H4 )NNBPh3 ] 3 and [Cp*2 Cr][Ph2 CNNHBPh3 ] 4. Computations showed both reactions proceed via transient radical anions of the diphenyldiazomethane-borane adducts to effect C-H bond activations.
Keywords: DFT; borane; diazomethane; one-electron reduction; radical anions.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

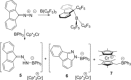

Similar articles
-
1,1-Hydroboration and a Borane Adduct of Diphenyldiazomethane: A Potential Prelude to FLP-N2 Chemistry.Angew Chem Int Ed Engl. 2017 Dec 22;56(52):16588-16592. doi: 10.1002/anie.201710337. Epub 2017 Dec 5. Angew Chem Int Ed Engl. 2017. PMID: 29108092
-
Zinc-Containing Radical Anions via Single Electron Transfer to Donor-Acceptor Adducts.Chemistry. 2018 Mar 15;24(16):3980-3983. doi: 10.1002/chem.201800607. Epub 2018 Feb 27. Chemistry. 2018. PMID: 29411915
-
Synthesis, structure, and reactivity of diazene adducts: isolation of iso-diazene stabilized as a borane adduct.Chemistry. 2014 Sep 8;20(37):11800-11. doi: 10.1002/chem.201402921. Epub 2014 Jul 24. Chemistry. 2014. PMID: 25059989
-
Influence of cyclopentadienyl ring-tilt on electron-transfer reactions: redox-induced reactivity of strained [2] and [3]ruthenocenophanes.Chemistry. 2014 Dec 1;20(49):16216-27. doi: 10.1002/chem.201403512. Epub 2014 Oct 8. Chemistry. 2014. PMID: 25298226
-
One- and Two-Electron Transfer Oxidation of 1,4-Disilabenzene with Formation of Stable Radical Cations and Dications.Chemistry. 2022 Jan 24;28(5):e202103715. doi: 10.1002/chem.202103715. Epub 2021 Dec 16. Chemistry. 2022. PMID: 34837718 Free PMC article.
Cited by
-
Reactions of Frustrated Lewis Pairs with Chloro-Diazirines: Cleavage of N=N Double Bonds.Angew Chem Int Ed Engl. 2022 Sep 12;61(37):e202209241. doi: 10.1002/anie.202209241. Epub 2022 Aug 3. Angew Chem Int Ed Engl. 2022. PMID: 35830598 Free PMC article.
-
Coordination of Al(C6F5)3 vs. B(C6F5)3 on group 6 end-on dinitrogen complexes: chemical and structural divergences.Chem Sci. 2024 Jun 17;15(29):11321-11336. doi: 10.1039/d4sc02713b. eCollection 2024 Jul 24. Chem Sci. 2024. PMID: 39055009 Free PMC article.
-
BNN-1,3-dipoles: isolation and intramolecular cycloaddition with unactivated arenes.Chem Sci. 2020 Jun 19;11(27):7053-7059. doi: 10.1039/d0sc02162h. Chem Sci. 2020. PMID: 34122998 Free PMC article.
-
An orbitally adapted push-pull template for N2 activation and reduction to diazene-diide.Chem Sci. 2023 Nov 21;14(48):14262-14270. doi: 10.1039/d3sc04390h. eCollection 2023 Dec 13. Chem Sci. 2023. PMID: 38098710 Free PMC article.
-
Reductive Cleavage of Dioxygen Mediated by a Lewis Superacidic Bis(borane).JACS Au. 2025 Jun 12;5(7):3032-3038. doi: 10.1021/jacsau.5c00516. eCollection 2025 Jul 28. JACS Au. 2025. PMID: 40747070 Free PMC article.
References
-
- Stephan D. W., Science 2016, 354, aaf7229. - PubMed
-
- Welch G. C., Juan R. R. S., Masuda J. D., Stephan D. W., Science 2006, 314, 1124–1126. - PubMed
-
- None
-
- McCahill J. S. J., Welch G. C., Stephan D. W., Angew. Chem. Int. Ed. 2007, 46, 4968–4971; - PubMed
- Angew. Chem. 2007, 119, 5056–5059;
-
- Dureen M. A., Brown C. C., Stephan D. W., Organometallics 2010, 29, 6594–6607;
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

