Combining immunoprofiling with machine learning to assess the effects of adjuvant formulation on human vaccine-induced immunity
- PMID: 31589550
- PMCID: PMC7062453
- DOI: 10.1080/21645515.2019.1654807
Combining immunoprofiling with machine learning to assess the effects of adjuvant formulation on human vaccine-induced immunity
Abstract
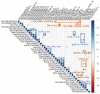
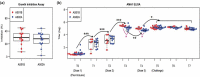
Adjuvants produce complex, but often subtle, effects on vaccine-induced immune responses that, nonetheless, play a critical role in vaccine efficacy. In-depth profiling of vaccine-induced cytokine, cellular, and antibody responses ("immunoprofiling") combined with machine-learning holds the promise of identifying adjuvant-specific immune response characteristics that can guide rational adjuvant selection. Here, we profiled human immune responses induced by vaccines adjuvanted with two similar, clinically relevant adjuvants, AS01B and AS02A, and identified key distinguishing characteristics, or immune signatures, they imprint on vaccine-induced immunity. Samples for this side-by-side comparison were from malaria-naïve individuals who had received a recombinant malaria subunit vaccine (AMA-1) that targets the pre-erythrocytic stage of the parasite. Both adjuvant formulations contain the same immunostimulatory components, QS21 and MPL, thus this study reveals the subtle impact that adjuvant formulation has on immunogenicity. Adjuvant-mediated immune signatures were established through a two-step approach: First, we generated a broad immunoprofile (serological, functional and cellular characterization of vaccine-induced responses). Second, we integrated the immunoprofiling data and identify what combination of immune features was most clearly able to distinguish vaccine-induced responses by adjuvant using machine learning. The computational analysis revealed statistically significant differences in cellular and antibody responses between cohorts and identified a combination of immune features that was able to distinguish subjects by adjuvant with 71% accuracy. Moreover, the in-depth characterization demonstrated an unexpected induction of CD8+ T cells by the recombinant subunit vaccine, which is rare and highly relevant for future vaccine design.
Keywords: Apical Membrane Antigen; Immunoprofile; Plasmodium falciparum; adjuvant; protection; vaccine.
Figures




References
-
- Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, Holman LA, James ER, Billingsley PF, Gunasekera A, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013;341:1359–65. - PubMed
-
- Tamminga C, Sedegah M, Maiolatesi S, Fedders C, Reyes S, Reyes A, Vasquez C, Alcorta Y, Chuang I, Spring M, et al. Human adenovirus 5-vectored Plasmodium falciparum NMRC-M3V-Ad-PfCA vaccine encoding CSP and AMA1 is safe, well-tolerated and immunogenic but does not protect against controlled human malaria infection. Hum Vaccin Immunother. 2013;9:2165–77. doi:10.4161/hv.24941. - DOI - PMC - PubMed
-
- Tamminga C, Sedegah M, Regis D, Chuang I, Epstein JE, Spring M, Mendoza-Silveiras J, McGrath S, Maiolatesi S, Reyes S, et al. Adenovirus-5-vectored P. falciparum vaccine expressing CSP and AMA1. Part B: safety, immunogenicity and protective efficacy of the CSP component. PLoS One. 2011;6:e25868. doi:10.1371/journal.pone.0025868. - DOI - PMC - PubMed
-
- Regules JA, Cicatelli SB, Bennett JW, Paolino KM, Twomey PS, Moon JE, Kathcart AK, Hauns KD, Komisar JL, Qabar AN, et al. Fractional third and fourth dose of RTS,S/AS01 malaria candidate vaccine: a phase 2a controlled human malaria parasite infection and immunogenicity study. J Infect Dis. 2016;214:762–71. doi:10.1093/infdis/jiw237. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
