Randomized controlled trial of cryotherapy to prevent paclitaxel-induced peripheral neuropathy (RU221511I); an ACCRU trial
- PMID: 31590108
- PMCID: PMC7558814
- DOI: 10.1016/j.breast.2019.09.011
Randomized controlled trial of cryotherapy to prevent paclitaxel-induced peripheral neuropathy (RU221511I); an ACCRU trial
Abstract
Purpose: This pilot trial aimed to assess if cooling hands and feet with crushed ice during receipt of paclitaxel helps prevent peripheral neuropathy.
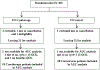
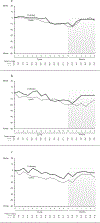
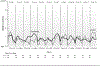
Methods: This prospective, randomized trial compared cryotherapy to standard care in patients initiating paclitaxel weekly x 12. For those on cryotherapy, hands and feet were cooled starting 15 min prior to and ending 15 min after each paclitaxel dose. EORTC QLQ-CIPN20 was completed at baseline, weekly x12, then monthly x6. Area under the curve (AUC) was calculated for subscale scores, adjusting for baseline, and compared between arms (Wilcoxon rank-sum test). Cross-study comparisons used data from 2 prior similarly-conducted neuropathy trials.
Results: Forty-six patients were accrued. Three withdrew and one was ineligible. Of the remaining 42 (21 cryotherapy, 21 control), 39 (19 cryotherapy, 20 control) were analyzable for AUC. Cryotherapy was well tolerated, but the AUC of the CIPN20 sensory scores over 12 weeks of paclitaxel was not found to differ between the study arms (mean difference 3.45, 95% CI -3.13 to 10.02, p = 0.26). However, the control arm of the current trial experienced less neuropathy than did the placebo arms of two previous similar trials. When our cryotherapy arm was compared to the combined control arms from all three trials, the cryotherapy arm had less neuropathy (Wilcoxon Rank-Sum p = 0.01).
Conclusion: While there was no difference in CIPN20 scores identified between the 2 study arms in the current phase II trial, further investigation is needed given that the control arm experienced less neuropathy than was expected.
Keywords: Chemotherapy-induced neuropathy; Cryotherapy; Paclitaxel acute pain syndrome; Paclitaxel-associated neuropathy.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of Interest Statement:
CLL reports grant funding from Breast Cancer Research Foundation during the conduct of this study and a consultant/advisory role with PledPharma, Metys, Disarm Therapeutics, and Asahi – all regarding efforts to reduce chemotherapy-induced neuropathy. MEL reports personal fees and non-financial support, outside the submitted work and Consulting for MERCK SHARP & DOHME CORPORATION, GALDERMA, JANSSEN RESEARCH & DEVELOPMENT, LLC, ABBVIE, INC., HELSINN HEALTHCARE SA, NOVOCURE INC, BOEHRINGER INGELHEIM PHARMA GMBH & CO.KG, F. HOFFMANN-LA ROCHE AG, ALLERGAN INC., AMGEN INC., E.R. SQUIBB & SONS, L.L.C., NOVARTIS PHARMACEUTICALS CORPORATION, EMD SERONO, INC., ASTRAZENECA PHARMACEUTICALS LP, GENENTECH, INC, LEO PHARMA INC, SEATTLE GENETICS, DEBIOPHARM, LINDI, BAYER, MANNER SAS, MENLO THER, CELLDEX, ABBVIE, LUTRIS, PIERRE FABRE, LEGACY HEALTHCARE, ROCHE, AMRYT PHARMA, JOHNSON & JOHNSON, PAXMAN COOLERS, ADJUCARE, DIGNITANA, BIOTECHSPERT, PAREXEL, and ADGERO. All other authors declare that they have no conflict of interest.
Figures



Similar articles
-
Comparison of the efficacy of cryotherapy and compression therapy for preventing nanoparticle albumin-bound paclitaxel-induced peripheral neuropathy: A prospective self-controlled trial.Breast. 2020 Feb;49:219-224. doi: 10.1016/j.breast.2019.12.011. Epub 2019 Dec 20. Breast. 2020. PMID: 31901783 Free PMC article. Clinical Trial.
-
Can pregabalin prevent paclitaxel-associated neuropathy?--An ACCRU pilot trial.Support Care Cancer. 2016 Feb;24(2):547-553. doi: 10.1007/s00520-015-2807-5. Epub 2015 Jul 9. Support Care Cancer. 2016. PMID: 26155765 Clinical Trial.
-
Cold therapy to prevent paclitaxel-induced peripheral neuropathy.Support Care Cancer. 2018 Oct;26(10):3461-3469. doi: 10.1007/s00520-018-4199-9. Epub 2018 Apr 21. Support Care Cancer. 2018. PMID: 29681015 Clinical Trial.
-
A meta-analysis of the utility of cryotherapy for preventing peripheral neuropathy among breast cancer patients receiving paclitaxel and nab-paclitaxel.Breast Cancer Res Treat. 2025 Apr;210(3):595-604. doi: 10.1007/s10549-024-07597-z. Epub 2025 Jan 19. Breast Cancer Res Treat. 2025. PMID: 39827229
-
Cryotherapy for prevention of chemotherapy induced peripheral neuropathy in breast cancer.Crit Rev Oncol Hematol. 2024 Feb;194:104244. doi: 10.1016/j.critrevonc.2023.104244. Epub 2023 Dec 20. Crit Rev Oncol Hematol. 2024. PMID: 38135017 Review.
Cited by
-
Wellbeing and Complementary Therapies in Breast Cancer Peripheral Neuropathy Care: A Scoping Review Focused on Foot Health.Cancers (Basel). 2023 Mar 31;15(7):2110. doi: 10.3390/cancers15072110. Cancers (Basel). 2023. PMID: 37046771 Free PMC article.
-
Innovative Approach for the Prevention of Chemotherapy-Induced Peripheral Neuropathy in Cancer Patients: A Pilot Study With the Hilotherm Device, the Poliambulanza Hospital Experience.Integr Cancer Ther. 2020 Jan-Dec;19:1534735420943287. doi: 10.1177/1534735420943287. Integr Cancer Ther. 2020. PMID: 32856475 Free PMC article.
-
Prevention of Oxaliplatin-Induced Peripheral Neuropathy: A Systematic Review and Meta-Analysis.Front Oncol. 2022 Feb 3;12:731223. doi: 10.3389/fonc.2022.731223. eCollection 2022. Front Oncol. 2022. PMID: 35186722 Free PMC article.
-
A Systematic Review and Meta-Analysis of the Effectiveness of Neuroprotectants for Paclitaxel-Induced Peripheral Neuropathy.Front Oncol. 2022 Jan 5;11:763229. doi: 10.3389/fonc.2021.763229. eCollection 2021. Front Oncol. 2022. PMID: 35070969 Free PMC article.
-
Safety and feasibility of concomitant scalp cooling and limb cryocompression to prevent paclitaxel-induced alopecia and neuropathy.Support Care Cancer. 2025 Feb 12;33(3):180. doi: 10.1007/s00520-024-08982-6. Support Care Cancer. 2025. PMID: 39937296 Free PMC article.
References
-
- Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941–1967. - PubMed
-
- Hershman DL, Weimer LH, Wang A, et al. Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res Treat. 2011;125(3):767–774. - PubMed
-
- Loprinzi CL, Maddocks-Christianson K, Wolf SL, et al. The Paclitaxel acute pain syndrome: sensitization of nociceptors as the putative mechasnism. Cancer J. 2007;13(6):399–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

