The Role of Mitochondria in the Mechanisms of Cardiac Ischemia-Reperfusion Injury
- PMID: 31590423
- PMCID: PMC6826663
- DOI: 10.3390/antiox8100454
The Role of Mitochondria in the Mechanisms of Cardiac Ischemia-Reperfusion Injury
Abstract
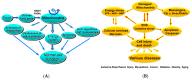
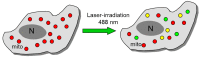
Mitochondria play a critical role in maintaining cellular function by ATP production. They are also a source of reactive oxygen species (ROS) and proapoptotic factors. The role of mitochondria has been established in many aspects of cell physiology/pathophysiology, including cell signaling. Mitochondria may deteriorate under various pathological conditions, including ischemia-reperfusion (IR) injury. Mitochondrial injury can be one of the main causes for cardiac and other tissue injuries by energy stress and overproduction of toxic reactive oxygen species, leading to oxidative stress, elevated calcium and apoptotic and necrotic cell death. However, the interplay among these processes in normal and pathological conditions is still poorly understood. Mitochondria play a critical role in cardiac IR injury, where they are directly involved in several pathophysiological mechanisms. We also discuss the role of mitochondria in the context of mitochondrial dynamics, specializations and heterogeneity. Also, we wanted to stress the existence of morphologically and functionally different mitochondrial subpopulations in the heart that may have different sensitivities to diseases and IR injury. Therefore, various cardioprotective interventions that modulate mitochondrial stability, dynamics and turnover, including various pharmacologic agents, specific mitochondrial antioxidants and uncouplers, and ischemic preconditioning can be considered as the main strategies to protect mitochondrial and cardiovascular function and thus enhance longevity.
Keywords: cytoskeleton; energy metabolism; heart; ischemia-reperfusion; mitochondria; mitochondrial heterogeneity; preconditioning; reactive oxygen species; signaling.
Conflict of interest statement
None declared.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

