Splenic Innate B1 B Cell Plasmablasts Produce Sustained Granulocyte-Macrophage Colony-Stimulating Factor and Interleukin-3 Cytokines during Murine Malaria Infections
- PMID: 31591168
- PMCID: PMC6867857
- DOI: 10.1128/IAI.00482-19
Splenic Innate B1 B Cell Plasmablasts Produce Sustained Granulocyte-Macrophage Colony-Stimulating Factor and Interleukin-3 Cytokines during Murine Malaria Infections
Abstract
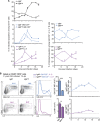
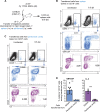
The physiopathology of malaria, one of the most deadly human parasitic diseases worldwide, is complex, as it is a systemic disease involving multiple parasitic stages and hosts and leads to the activation of numerous immune cells and release of inflammatory mediators. While some cytokines increased in the blood of patients infected with Plasmodium falciparum have been extensively studied, others, such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-3 (IL-3), have not received much attention. GM-CSF and IL-3 belong to the β common (βc/CD131) chain family of cytokines, which exhibit pleiotropic functions, including the regulation of myeloid cell growth, differentiation, and activation. GM-CSF can be secreted by multiple cell types, whereas IL-3 is mostly restricted to T cells, yet innate response activator (IRA) B cells, a subset of innate B1 B cells, also produce significant amounts of these cytokines during bacterial sepsis via Toll-like receptor 4 (TLR4)/MyD88 sensing of lipopolysaccharides. Herein, using murine models of malaria, we report a sustained production of GM-CSF and IL-3 from IgM+ and IgM-/IgG+ CD138+ Blimp-1+ innate B1b B cell plasmablasts. IgM+ B1b B cells include IRA-like and non-IRA B cells and express higher levels of both cytokines than do their IgG+ counterparts. Interestingly, as infection progresses, the relative proportion of IgM+ B1 B cells decreases while that of IgG+ plasmablasts increases, correlating with potential isotype switching of GM-CSF- and IL-3-producing IgM+ B1 B cells. GM-CSF/IL-3+ B1 B cells originate in the spleen of infected mice and are partially dependent on type I and type II interferon signaling to produce both cytokines. These data reveal that GM-CSF and IL-3 are produced during malaria infections, initially from IgM+ and then from IgG+ B1b B cell plasmablasts, which may represent important emergency cellular sources of these cytokines. These results further highlight the phenotypic heterogeneity of innate B1 B cell subsets and of their possible fates in a relevant murine model of parasitic infection in vivo.
Keywords: B1 B cells; GM-CSF; IL-3; IRA B cells; Plasmodium; interferon gamma; malaria; plasmablasts; type I interferon.
Copyright © 2019 American Society for Microbiology.
Figures




References
-
- Chaussabel D, Pajak B, Vercruysse V, Bisseye C, Garze V, Habib M, Goldman M, Moser M, Vray B. 2003. Alteration of migration and maturation of dendritic cells and T-cell depletion in the course of experimental Trypanosoma cruzi infection. Lab Invest 83:1373–1382. doi:10.1097/01.LAB.0000087587.93781.6F. - DOI - PubMed
-
- Crompton PD, Moebius J, Portugal S, Waisberg M, Hart G, Garver LS, Miller LH, Barillas-Mury C, Pierce SK. 2014. Malaria immunity in man and mosquito: insights into unsolved mysteries of a deadly infectious disease. Annu Rev Immunol 32:157–187. doi:10.1146/annurev-immunol-032713-120220. - DOI - PMC - PubMed
-
- Spaulding E, Fooksman D, Moore JM, Saidi A, Feintuch CM, Reizis B, Chorro L, Daily J, Lauvau G. 2016. STING-licensed macrophages prime type I IFN production by plasmacytoid dendritic cells in the bone marrow during severe Plasmodium yoelii malaria. PLoS Pathog 12:e1005975. doi:10.1371/journal.ppat.1005975. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

