Screening Legionella effectors for antiviral effects reveals Rab1 GTPase as a proviral factor coopted for tombusvirus replication
- PMID: 31591191
- PMCID: PMC6815150
- DOI: 10.1073/pnas.1911108116
Screening Legionella effectors for antiviral effects reveals Rab1 GTPase as a proviral factor coopted for tombusvirus replication
Abstract
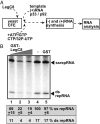
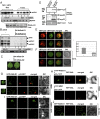
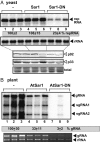
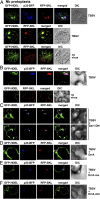
Bacterial virulence factors or effectors are proteins targeted into host cells to coopt or interfere with cellular proteins and pathways. Viruses often coopt the same cellular proteins and pathways to support their replication in infected cells. Therefore, we screened the Legionella pneumophila effectors to probe virus-host interactions and identify factors that modulate tomato bushy stunt virus (TBSV) replication in yeast surrogate host. Among 302 Legionella effectors tested, 28 effectors affected TBSV replication. To unravel a coopted cellular pathway in TBSV replication, the identified DrrA effector from Legionella was further exploited. We find that expression of DrrA in yeast or plants blocks TBSV replication through inhibiting the recruitment of Rab1 small GTPase and endoplasmic reticulum-derived COPII vesicles into the viral replication compartment. TBSV hijacks Rab1 and COPII vesicles to create enlarged membrane surfaces and optimal lipid composition within the viral replication compartment. To further validate our Legionella effector screen, we used the Legionella effector LepB lipid kinase to confirm the critical proviral function of PI(3)P phosphoinositide and the early endosomal compartment in TBSV replication. We demonstrate the direct inhibitory activity of LegC8 effector on TBSV replication using a cell-free replicase reconstitution assay. LegC8 inhibits the function of eEF1A, a coopted proviral host factor. Altogether, the identified bacterial effectors with anti-TBSV activity could be powerful reagents in cell biology and virus-host interaction studies. This study provides important proof of concept that bacterial effector proteins can be a useful toolbox to identify host factors and cellular pathways coopted by (+)RNA viruses.
Keywords: effector; host factor; tomato bushy stunt virus; viral replication; yeast.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
Screening bacterial effectors and human virus proteins in yeast to identify host factors driving tombusvirus RNA recombination: a role for autophagy and membrane phospholipid content.J Virol. 2025 Jun 17;99(6):e0166124. doi: 10.1128/jvi.01661-24. Epub 2025 May 27. J Virol. 2025. PMID: 40422074 Free PMC article.
-
Co-opted Cellular Sac1 Lipid Phosphatase and PI(4)P Phosphoinositide Are Key Host Factors during the Biogenesis of the Tombusvirus Replication Compartment.J Virol. 2020 Jun 1;94(12):e01979-19. doi: 10.1128/JVI.01979-19. Print 2020 Jun 1. J Virol. 2020. PMID: 32269127 Free PMC article.
-
Reconstitution of an RNA Virus Replicase in Artificial Giant Unilamellar Vesicles Supports Full Replication and Provides Protection for the Double-Stranded RNA Replication Intermediate.J Virol. 2020 Aug 31;94(18):e00267-20. doi: 10.1128/JVI.00267-20. Print 2020 Aug 31. J Virol. 2020. PMID: 32641477 Free PMC article.
-
Cell-Free and Cell-Based Approaches to Explore the Roles of Host Membranes and Lipids in the Formation of Viral Replication Compartment Induced by Tombusviruses.Viruses. 2016 Mar 3;8(3):68. doi: 10.3390/v8030068. Viruses. 2016. PMID: 26950140 Free PMC article. Review.
-
Tombusvirus-Host Interactions: Co-Opted Evolutionarily Conserved Host Factors Take Center Court.Annu Rev Virol. 2016 Sep 29;3(1):491-515. doi: 10.1146/annurev-virology-110615-042312. Epub 2016 Aug 8. Annu Rev Virol. 2016. PMID: 27578441 Review.
Cited by
-
Manipulation of the Cellular Membrane-Cytoskeleton Network for RNA Virus Replication and Movement in Plants.Viruses. 2023 Mar 14;15(3):744. doi: 10.3390/v15030744. Viruses. 2023. PMID: 36992453 Free PMC article. Review.
-
Screening bacterial effectors and human virus proteins in yeast to identify host factors driving tombusvirus RNA recombination: a role for autophagy and membrane phospholipid content.J Virol. 2025 Jun 17;99(6):e0166124. doi: 10.1128/jvi.01661-24. Epub 2025 May 27. J Virol. 2025. PMID: 40422074 Free PMC article.
-
Subversion of selective autophagy for the biogenesis of tombusvirus replication organelles inhibits autophagy.PLoS Pathog. 2024 Mar 14;20(3):e1012085. doi: 10.1371/journal.ppat.1012085. eCollection 2024 Mar. PLoS Pathog. 2024. PMID: 38484009 Free PMC article.
-
Mobilization of nuclear antiviral factors by exportin XPO1 via the actin network inhibits RNA virus replication.PLoS Pathog. 2025 Aug 19;21(8):e1012841. doi: 10.1371/journal.ppat.1012841. eCollection 2025 Aug. PLoS Pathog. 2025. PMID: 40828816 Free PMC article.
-
Legionella pneumophila, a Rosetta stone to understanding bacterial pathogenesis.J Bacteriol. 2024 Dec 19;206(12):e0032424. doi: 10.1128/jb.00324-24. Epub 2024 Dec 5. J Bacteriol. 2024. PMID: 39636264 Free PMC article. Review.
References
-
- Wang A., Dissecting the molecular network of virus-plant interactions: The complex roles of host factors. Annu. Rev. Phytopathol. 53, 45–66 (2015). - PubMed
-
- den Boon J. A., Ahlquist P., Organelle-like membrane compartmentalization of positive-strand RNA virus replication factories. Annu. Rev. Microbiol. 64, 241–256 (2010). - PubMed
-
- Diaz A., Wang X., Bromovirus-induced remodeling of host membranes during viral RNA replication. Curr. Opin. Virol. 9, 104–110 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

