Lipid-targeting pleckstrin homology domain turns its autoinhibitory face toward the TEC kinases
- PMID: 31591208
- PMCID: PMC6815127
- DOI: 10.1073/pnas.1907566116
Lipid-targeting pleckstrin homology domain turns its autoinhibitory face toward the TEC kinases
Abstract
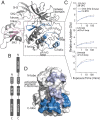
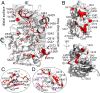
The pleckstrin homology (PH) domain is well known for its phospholipid targeting function. The PH-TEC homology (PHTH) domain within the TEC family of tyrosine kinases is also a crucial component of the autoinhibitory apparatus. The autoinhibitory surface on the PHTH domain has been previously defined, and biochemical investigations have shown that PHTH-mediated inhibition is mutually exclusive with phosphatidylinositol binding. Here we use hydrogen/deuterium exchange mass spectrometry, nuclear magnetic resonance (NMR), and evolutionary sequence comparisons to map where and how the PHTH domain affects the Bruton's tyrosine kinase (BTK) domain. The data map a PHTH-binding site on the activation loop face of the kinase C lobe, suggesting that the PHTH domain masks the activation loop and the substrate-docking site. Moreover, localized NMR spectral changes are observed for non-surface-exposed residues in the active site and on the distal side of the kinase domain. These data suggest that the association of PHTH induces allosteric conformational shifts in regions of the kinase domain that are critical for catalysis. Through statistical comparisons of diverse tyrosine kinase sequences, we identify residues unique to BTK that coincide with the experimentally determined PHTH-binding surface on the kinase domain. Our data provide a more complete picture of the autoinhibitory conformation adopted by full-length TEC kinases, creating opportunities to target the regulatory domains to control the function of these kinases in a biological setting.
Keywords: PH domain; kinase; regulation.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: A.H.A. has an equity interest in ImmVue Therapeutics, Inc., a company that may potentially benefit from the research results. The terms of this arrangement have been reviewed and approved by Iowa State University in accordance with its conflict of interest policies.
Figures



References
-
- Andreotti A. H., Joseph R. E., Conley J. M., Iwasa J., Berg L. J., Multidomain control over TEC kinase activation state tunes the T cell response. Annu. Rev. Immunol. 36, 549–578 (2018). - PubMed
-
- Berg L. J., Finkelstein L. D., Lucas J. A., Schwartzberg P. L., Tec family kinases in T lymphocyte development and function. Annu. Rev. Immunol. 23, 549–600 (2005). - PubMed
-
- Okoh M. P., Vihinen M., Pleckstrin homology domains of tec family protein kinases. Biochem. Biophys. Res. Commun. 265, 151–157 (1999). - PubMed
-
- Saito K., Scharenberg A. M., Kinet J. P., Interaction between the Btk PH domain and phosphatidylinositol-3,4,5-trisphosphate directly regulates Btk. J. Biol. Chem. 276, 16201–16206 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

