Molecular profiling predicts meningioma recurrence and reveals loss of DREAM complex repression in aggressive tumors
- PMID: 31591222
- PMCID: PMC6815170
- DOI: 10.1073/pnas.1912858116
Molecular profiling predicts meningioma recurrence and reveals loss of DREAM complex repression in aggressive tumors
Abstract
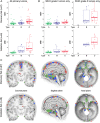
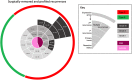
Meningiomas account for one-third of all primary brain tumors. Although typically benign, about 20% of meningiomas are aggressive, and despite the rigor of the current histopathological classification system there remains considerable uncertainty in predicting tumor behavior. Here, we analyzed 160 tumors from all 3 World Health Organization (WHO) grades (I through III) using clinical, gene expression, and sequencing data. Unsupervised clustering analysis identified 3 molecular types (A, B, and C) that reliably predicted recurrence. These groups did not directly correlate with the WHO grading system, which classifies more than half of the tumors in the most aggressive molecular type as benign. Transcriptional and biochemical analyses revealed that aggressive meningiomas involve loss of the repressor function of the DREAM complex, which results in cell-cycle activation; only tumors in this category tend to recur after full resection. These findings should improve our ability to predict recurrence and develop targeted treatments for these clinically challenging tumors.
Keywords: NF2; PRC2; brain tumor; classification; oncogenesis.
Conflict of interest statement
Competing interest statement: S.E.P. is a member of the Scientific Advisory Board for Baylor Genetics.
Figures




References
-
- Pearson B. E., et al. , Hitting a moving target: Evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg. Focus 24, E3 (2008). - PubMed
-
- Aghi M. K., et al. , Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery 64, 56–60, discussion 60 (2009). - PubMed
-
- Modha A., Gutin P. H., Diagnosis and treatment of atypical and anaplastic meningiomas: A review. Neurosurgery 57, 538–550, discussion 538–550 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

