Cyclin D1 is Associated with Radiosensitivity of Triple-Negative Breast Cancer Cells to Proton Beam Irradiation
- PMID: 31591311
- PMCID: PMC6801441
- DOI: 10.3390/ijms20194943
Cyclin D1 is Associated with Radiosensitivity of Triple-Negative Breast Cancer Cells to Proton Beam Irradiation
Abstract
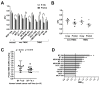
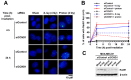
Proton therapy offers a distinct physical advantage over conventional X-ray therapy, but its biological advantages remain understudied. In this study, we aimed to identify genetic factors that contribute to proton sensitivity in breast cancer (BC). Therefore, we screened relative biological effectiveness (RBE) of 230 MeV protons, compared to 6 MV X-rays, in ten human BC cell lines, including five triple-negative breast cancer (TNBC) cell lines. Clonogenic survival assays revealed a wide range of proton RBE across the BC cell lines, with one out of ten BC cell lines having an RBE significantly different from the traditional generic RBE of 1.1. An abundance of cyclin D1 was associated with proton RBE. Downregulation of RB1 by siRNA or a CDK4/6 inhibitor increased proton sensitivity but not proton RBE. Instead, the depletion of cyclin D1 increased proton RBE in two TNBC cell lines, including MDA-MB-231 and Hs578T cells. Conversely, overexpression of cyclin D1 decreased the proton RBE in cyclin D1-deficient BT-549 cells. The depletion of cyclin D1 impaired proton-induced RAD51 foci formation in MDA-MB-231 cells. Taken together, this study provides important clues about the cyclin D1-CDK4-RB1 pathway as a potential target for proton beam therapy in TNBC.
Keywords: CDK4/6 inhibitor; breast cancer; cyclin D1; proton therapy; relative biological effectiveness.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Lung cancer cell line screen links fanconi anemia/BRCA pathway defects to increased relative biological effectiveness of proton radiation.Int J Radiat Oncol Biol Phys. 2015 Apr 1;91(5):1081-9. doi: 10.1016/j.ijrobp.2014.12.046. Int J Radiat Oncol Biol Phys. 2015. PMID: 25832698
-
Checkpoint Kinase 1 (CHK1) Inhibition Enhances the Sensitivity of Triple-Negative Breast Cancer Cells to Proton Irradiation via Rad51 Downregulation.Int J Mol Sci. 2020 Apr 13;21(8):2691. doi: 10.3390/ijms21082691. Int J Mol Sci. 2020. PMID: 32294924 Free PMC article.
-
Induction of AMPK activation by N,N'-diarylurea FND-4b decreases growth and increases apoptosis in triple negative and estrogen-receptor positive breast cancers.PLoS One. 2019 Mar 15;14(3):e0209392. doi: 10.1371/journal.pone.0209392. eCollection 2019. PLoS One. 2019. PMID: 30875375 Free PMC article.
-
Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer.Phys Med Biol. 2014 Nov 21;59(22):R419-72. doi: 10.1088/0031-9155/59/22/R419. Epub 2014 Oct 31. Phys Med Biol. 2014. PMID: 25361443 Review.
-
Acquired radioresistance of cancer and the AKT/GSK3β/cyclin D1 overexpression cycle.J Radiat Res. 2011;52(5):539-44. doi: 10.1269/jrr.11098. Epub 2011 Sep 1. J Radiat Res. 2011. PMID: 21881296 Review.
Cited by
-
Rab14 Overexpression Promotes Proliferation and Invasion Through YAP Signaling in Non-Small Cell Lung Cancers.Onco Targets Ther. 2020 Sep 18;13:9269-9280. doi: 10.2147/OTT.S255644. eCollection 2020. Onco Targets Ther. 2020. PMID: 32982313 Free PMC article.
-
Molecular Investigation on a Triple Negative Breast Cancer Xenograft Model Exposed to Proton Beams.Int J Mol Sci. 2020 Sep 1;21(17):6337. doi: 10.3390/ijms21176337. Int J Mol Sci. 2020. PMID: 32882850 Free PMC article.
-
Isoliquiritigenin Derivative Regulates miR-374a/BAX Axis to Suppress Triple-Negative Breast Cancer Tumorigenesis and Development.Front Pharmacol. 2020 Mar 31;11:378. doi: 10.3389/fphar.2020.00378. eCollection 2020. Front Pharmacol. 2020. PMID: 32296334 Free PMC article.
-
RB expression confers sensitivity to CDK4/6 inhibitor-mediated radiosensitization across breast cancer subtypes.JCI Insight. 2022 Feb 8;7(3):e154402. doi: 10.1172/jci.insight.154402. JCI Insight. 2022. PMID: 34932500 Free PMC article.
-
Heterogeneity in biological response of MDA-MB-231 cells after proton irradiation along different parts of the depth-dose curve: before, within, and behind the Bragg peak.Rep Pract Oncol Radiother. 2024 Oct 3;29(4):478-487. doi: 10.5603/rpor.102129. eCollection 2024. Rep Pract Oncol Radiother. 2024. PMID: 39895957 Free PMC article.
References
-
- Fisher B., Anderson S., Bryant J., Margolese R.G., Deutsch M., Fisher E.R., Jeong J.H., Wolmark N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

