Factors Influencing Persistence of Diphtheria Immunity and Immune Response to a Booster Dose in Healthy Slovak Adults
- PMID: 31591336
- PMCID: PMC6963617
- DOI: 10.3390/vaccines7040139
Factors Influencing Persistence of Diphtheria Immunity and Immune Response to a Booster Dose in Healthy Slovak Adults
Abstract
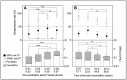
We assessed the long-term persistence of humoral immunity against diphtheria in adults with childhood vaccination and the immunogenicity of a booster dose considering demographic, behavioural and vaccinating factors. We conducted a trial in 200 healthy Slovak adults aged 24-65 years, immunised against diphtheria in childhood and against tetanus at regular 10-15 year intervals, and receiving a dose of a tetanus-diphtheria toxoid vaccine. The response was determined by ELISA antibody concentrations of paired sera before and at 4 weeks post-vaccination. A seroprotection rate of 21% (95% confidence interval, CI 15.6-27.3%) was found in adults up to 59 years since the last vaccination with seroprotective levels of antibodies against diphtheria ≥0.1 IU/mL and a geometric mean concentration of 0.05 IU/mL. Conversely, seropositive levels ≥0.01 IU/mL were observed in 98% of adults (95% CI 95-99.5%). Booster-induced seroprotection was achieved in 78% of adults (95% CI 71.6-83.5%) clearly depending on pre-booster antibody levels correlating with age and time since the last vaccination. Moreover, only 54.2% of smokers and 53.3% of patients on statins exhibited seroprotection. Booster vaccination against diphtheria was unable to confer seroprotection in all recipients of only childhood vaccination.
Keywords: diphtheria vaccination; immunity persistence; pre-vaccination levels; seroprotection rate; smoking; statins.
Conflict of interest statement
Marek Petráš, Milica Molitorisová, Jana Dáňová, Alexander Čelko, Elena Nováková, Mária Štefkovičová, Zuzana Krištúfková, Jana Malinová and Ivana Králová Lesná have no conflicts of interest to declare. Vladimir Oleár has received lecture fees from Biodrug s.r.o. (Slovakia), the study sponsor. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures
Similar articles
-
Antibody persistence and safety and immunogenicity of a second booster dose nine years after a first booster vaccination with a reduced antigen diphtheria-tetanus-acellular pertussis vaccine (Tdap) in adults.Vaccine. 2018 Oct 8;36(42):6325-6333. doi: 10.1016/j.vaccine.2018.08.051. Epub 2018 Sep 7. Vaccine. 2018. PMID: 30197282 Clinical Trial.
-
Decennial administration in young adults of a reduced-antigen content diphtheria, tetanus, acellular pertussis vaccine containing two different concentrations of aluminium.Vaccine. 2015 Jun 12;33(26):3026-34. doi: 10.1016/j.vaccine.2014.10.049. Epub 2015 Jan 19. Vaccine. 2015. PMID: 25613716 Clinical Trial.
-
Persistence of humoral immunity to tetanus and diphtheria in hematopoietic stem cell transplant recipients after post-transplant immunization.Pediatr Blood Cancer. 2012 Nov;59(5):908-13. doi: 10.1002/pbc.24186. Epub 2012 Apr 18. Pediatr Blood Cancer. 2012. PMID: 22514148 Clinical Trial.
-
Spotlight on DTPa-HBV-IPV/Hib Vaccine (Infanrix hexa).BioDrugs. 2010 Oct 1;24(5):299-302. doi: 10.2165/11206690-000000000-00000. BioDrugs. 2010. PMID: 20795752 Review.
-
Tdap5 vaccine (Covaxis): a review of its use as a single-booster immunization for the prevention of tetanus, diphtheria, and pertussis in children (aged 4 years), adolescents, and adults.BioDrugs. 2010 Dec 1;24(6):387-406. doi: 10.2165/11206000-000000000-00000. BioDrugs. 2010. PMID: 21043546 Review.
Cited by
-
How does geographical diversity shape vaccine efficacy?Clin Exp Vaccine Res. 2024 Oct;13(4):271-300. doi: 10.7774/cevr.2024.13.4.271. Epub 2024 Oct 31. Clin Exp Vaccine Res. 2024. PMID: 39525670 Free PMC article. Review.
-
Tetanus and Diphtheria Toxoid-Containing Vaccine in Multiple Sclerosis Patients: A Real-World Prospective, Open-Label, Multi-Centre Study.Vaccines (Basel). 2025 Apr 24;13(5):451. doi: 10.3390/vaccines13050451. Vaccines (Basel). 2025. PMID: 40432063 Free PMC article.
-
Diphtheria and Tetanus Immunity Status among Greek Adults: Results from a Nationwide Seroprevalence Study.Vaccines (Basel). 2024 Apr 2;12(4):378. doi: 10.3390/vaccines12040378. Vaccines (Basel). 2024. PMID: 38675760 Free PMC article.
-
The Effect of Smoking on Humoral Response to COVID-19 Vaccines: A Systematic Review of Epidemiological Studies.Vaccines (Basel). 2022 Feb 16;10(2):303. doi: 10.3390/vaccines10020303. Vaccines (Basel). 2022. PMID: 35214761 Free PMC article. Review.
References
-
- CDC Vaccine Administration. General Best Practice Guidelines for Immunization, Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP) [(accessed on 15 March 2019)]; Available online: https//www.cdc.gov/vaccines/hcp/acip-recs/general-recs/administration.ht....
-
- WHO Expert Committee on Biological Standardization Sixty-Third Report. WHO Technical Report Series No. 980. [(accessed on 21 June 2019)];2014 Available online: https//www.who.int/biologicals/WHO_TRS_980_WEB.pdf. - PubMed
LinkOut - more resources
Full Text Sources