The oncometabolite 2-hydroxyglutarate produced by mutant IDH1 sensitizes cells to ferroptosis
- PMID: 31591388
- PMCID: PMC6779886
- DOI: 10.1038/s41419-019-1984-4
The oncometabolite 2-hydroxyglutarate produced by mutant IDH1 sensitizes cells to ferroptosis
Abstract
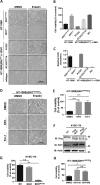
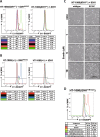
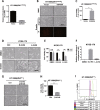
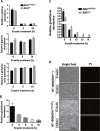
Ferroptosis is a non-apoptotic form of cell death characterized by the iron-dependent lipid peroxidation and is implicated in several human pathologies, such as tissue ischemia, neurodegeneration, and cancer. Ferroptosis appears to be high cell-context dependent and the regulation of ferroptosis by physiological or pathological conditions are unclear. Here, we report that tumor-derived IDH1 mutation sensitizes cells to ferroptosis. Deletion of the mutant IDH1 allele in IDH1 heterozygous tumor cells or pharmacological inhibition of mutant IDH1 to produce the oncometabolite D-2-hydroxyglutarate (D-2-HG) confers resistance to erastin-induced ferroptosis. Conversely, ectopic expression of mutant IDH1 or treatment of cells with cell-permeable D-2-HG promotes the accumulation of lipid reactive oxygen species (ROS) and subsequently ferroptosis. Mechanistically, mutant IDH1 reduces the protein level of the glutathione peroxidase 4 (GPX4), a key enzyme in removing lipid ROS and ferroptosis, and promotes depletion of glutathione. Our results uncover a new role of mutant IDH1 and 2-HG in ferroptosis.
Conflict of interest statement
Kun-Liang Guan is a co-founder of Vivace Therapeutics. Yue Xiong is a co-founder of Cullgen Inc. The remaining authors declare that they have no conflict of interest.
Figures
Similar articles
-
ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation.Cell Death Dis. 2019 Oct 28;10(11):822. doi: 10.1038/s41419-019-2064-5. Cell Death Dis. 2019. PMID: 31659150 Free PMC article.
-
Discovery of a Novel Chemical Scaffold Against Mutant Isocitrate Dehydrogenase 1 (IDH1).Anticancer Res. 2020 Sep;40(9):4929-4935. doi: 10.21873/anticanres.14496. Anticancer Res. 2020. PMID: 32878781
-
Lack of evidence for substrate channeling or flux between wildtype and mutant isocitrate dehydrogenase to produce the oncometabolite 2-hydroxyglutarate.J Biol Chem. 2018 Dec 28;293(52):20051-20061. doi: 10.1074/jbc.RA118.004278. Epub 2018 Oct 31. J Biol Chem. 2018. PMID: 30381394 Free PMC article.
-
Lipid Peroxidation-Dependent Cell Death Regulated by GPx4 and Ferroptosis.Curr Top Microbiol Immunol. 2017;403:143-170. doi: 10.1007/82_2016_508. Curr Top Microbiol Immunol. 2017. PMID: 28204974 Review.
-
The Relationship between Ferroptosis and Tumors: A Novel Landscape for Therapeutic Approach.Curr Gene Ther. 2019;19(2):117-124. doi: 10.2174/1566523219666190628152137. Curr Gene Ther. 2019. PMID: 31264548 Free PMC article. Review.
Cited by
-
Recent insights into the microRNA-dependent modulation of gliomas from pathogenesis to diagnosis and treatment.Cell Mol Biol Lett. 2022 Aug 3;27(1):65. doi: 10.1186/s11658-022-00354-4. Cell Mol Biol Lett. 2022. PMID: 35922753 Free PMC article. Review.
-
Transsulfuration pathway activation attenuates oxidative stress and ferroptosis in sickle primary erythroblasts and transgenic mice.Commun Biol. 2025 Jan 6;8(1):15. doi: 10.1038/s42003-024-07424-7. Commun Biol. 2025. PMID: 39762627 Free PMC article.
-
IDH1 Promotes Foam Cell Formation by Aggravating Macrophage Ferroptosis.Biology (Basel). 2022 Sep 23;11(10):1392. doi: 10.3390/biology11101392. Biology (Basel). 2022. PMID: 36290297 Free PMC article.
-
The Regulatory Mechanism and Research Progress of Ferroptosis in Gastric Cancer.Technol Cancer Res Treat. 2023 Jan-Dec;22:15330338231168498. doi: 10.1177/15330338231168498. Technol Cancer Res Treat. 2023. PMID: 37078206 Free PMC article. Review.
-
Ferroptosis and mitochondrial dysfunction in acute central nervous system injury.Front Cell Neurosci. 2023 Aug 9;17:1228968. doi: 10.3389/fncel.2023.1228968. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37622048 Free PMC article. Review.
References
-
- Soulinna EM, Winberg AM. 7-Ethoxycoumarin O-deethylation and methylumbelliferone conjugation in isolated fetal and adult bovine hepatocytes. Drug Metab. Disposition: Biol. Fate Chem. 1985;13:722–724. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

