Systematic review of the evidence on the cost-effectiveness of pharmacogenomics-guided treatment for cardiovascular diseases
- PMID: 31591509
- PMCID: PMC7056639
- DOI: 10.1038/s41436-019-0667-y
Systematic review of the evidence on the cost-effectiveness of pharmacogenomics-guided treatment for cardiovascular diseases
Abstract
Purpose: To examine the evidence on the cost-effectiveness of implementing pharmacogenomics (PGx) in cardiovascular disease (CVD) care.
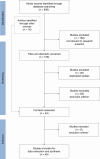
Methods: We conducted a systematic review using multiple databases from inception to 2018. The titles and abstracts of cost-effectiveness studies on PGx-guided treatment in CVD care were screened, and full texts were extracted.
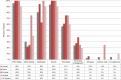
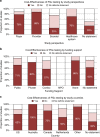
Results: We screened 909 studies and included 46 to synthesize. Acute coronary syndrome and atrial fibrillation were the predominantly studied conditions (59%). Most studies (78%) examined warfarin-CYP2C9/VKORC1 or clopidogrel-CYP2C19. A payer's perspective was commonly used (39%) for cost calculations, and most studies (46%) were US-based. The majority (67%) of the studies found PGx testing to be cost-effective in CVD care, but cost-effectiveness varied across drugs and conditions. Two studies examined PGx panel testing, of which one examined pre-emptive testing strategies.
Conclusion: We found mixed evidence on the cost-effectiveness of PGx in CVD care. Supportive evidence exists for clopidogrel-CYP2C19 and warfarin-CYP2C9/VKORC1, but evidence is limited in other drug-gene combinations. Gaps persist, including unclear explanation of perspective and cost inputs, underreporting of study design elements critical to economic evaluations, and limited examination of PGx panel and pre-emptive testing for their cost-effectiveness. This review identifies the need for further research on economic evaluations of PGx implementation.
Keywords: cardiovascular disease; cost-effectiveness; disease management; economic evaluation; pharmacogenomics.
Conflict of interest statement
None of the authors have any potential conflicts of interest with respect to the authorship and/or publication of this article. None of the authors reported any financial relationships with any organizations that might have an interest in the submitted work in the previous three years. RW and LW are co-founders of and stock-holders in OneOme LLC, a pharmacogenomic decision support company. There are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Weinshilboum R. Inheritance and drug response. N Engl J Med. 2003;348:529–537. - PubMed
-
- Weinshilboum R, Wang L. Pharmacogenomics: bench to bedside. Nat Rev Drug Discov. 2004;3:739. - PubMed
-
- Payne K, Gavan SP, Wright SJ, Thompson AJ. Cost-effectiveness analyses of genetic and genomic diagnostic tests. Nat Rev Genet. 2018;19:235. - PubMed
-
- World Health Organization. Cardiovacular diseases (CVDs). 2017. https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases....
-
- American Heart Assocation. Cardiovascular disease: a costly burden for America, projections through 2035. CVD Burden Report. 2017. https://healthmetrics.heart.org/wp-content/uploads/2017/10/Cardiovascula.... Accessed February 29, 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

