Locally instructed CXCR4hi neutrophils trigger environment-driven allergic asthma through the release of neutrophil extracellular traps
- PMID: 31591573
- PMCID: PMC6859073
- DOI: 10.1038/s41590-019-0496-9
Locally instructed CXCR4hi neutrophils trigger environment-driven allergic asthma through the release of neutrophil extracellular traps
Abstract
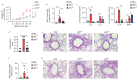
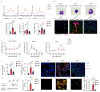
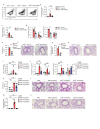
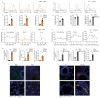
Low exposure to microbial products, respiratory viral infections and air pollution are major risk factors for allergic asthma, yet the mechanistic links between such conditions and host susceptibility to type 2 allergic disorders remain unclear. Through the use of single-cell RNA sequencing, we characterized lung neutrophils in mice exposed to a pro-allergic low dose of lipopolysaccharide (LPS) or a protective high dose of LPS before exposure to house dust mites. Unlike exposure to a high dose of LPS, exposure to a low dose of LPS instructed recruited neutrophils to upregulate their expression of the chemokine receptor CXCR4 and to release neutrophil extracellular traps. Low-dose LPS-induced neutrophils and neutrophil extracellular traps potentiated the uptake of house dust mites by CD11b+Ly-6C+ dendritic cells and type 2 allergic airway inflammation in response to house dust mites. Neutrophil extracellular traps derived from CXCR4hi neutrophils were also needed to mediate allergic asthma triggered by infection with influenza virus or exposure to ozone. Our study indicates that apparently unrelated environmental risk factors can shape recruited lung neutrophils to promote the initiation of allergic asthma.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

