Avoidable flaws in observational analyses: an application to statins and cancer
- PMID: 31591592
- PMCID: PMC7076561
- DOI: 10.1038/s41591-019-0597-x
Avoidable flaws in observational analyses: an application to statins and cancer
Abstract
The increasing availability of large healthcare databases is fueling an intense debate on whether real-world data should play a role in the assessment of the benefit-risk of medical treatments. In many observational studies, for example, statin users were found to have a substantially lower risk of cancer than in meta-analyses of randomized trials. Although such discrepancies are often attributed to a lack of randomization in the observational studies, they might be explained by flaws that can be avoided by explicitly emulating a target trial (the randomized trial that would answer the question of interest). Using the electronic health records of 733,804 UK adults, we emulated a target trial of statins and cancer and compared our estimates with those obtained using previously applied analytic approaches. Over the 10-yr follow-up, 28,408 individuals developed cancer. Under the target trial approach, estimated observational analogs of intention-to-treat and per-protocol 10-yr cancer-free survival differences were -0.5% (95% confidence interval (CI) -1.0%, 0.0%) and -0.3% (95% CI -1.5%, 0.5%), respectively. By contrast, previous analytic approaches yielded estimates that appeared to be strongly protective. Our findings highlight the importance of explicitly emulating a target trial to reduce bias in the effect estimates derived from observational analyses.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures

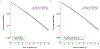
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

