Single-cell immune landscape of human atherosclerotic plaques
- PMID: 31591603
- PMCID: PMC7318784
- DOI: 10.1038/s41591-019-0590-4
Single-cell immune landscape of human atherosclerotic plaques
Abstract
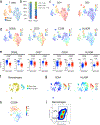
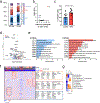
Atherosclerosis is driven by multifaceted contributions of the immune system within the circulation and at vascular focal sites. However, specific characteristics of dysregulated immune cells within atherosclerotic lesions that lead to clinical events such as ischemic stroke or myocardial infarction are poorly understood. Here, using single-cell proteomic and transcriptomic analyses, we uncovered distinct features of both T cells and macrophages in carotid artery plaques of patients with clinically symptomatic disease (recent stroke or transient ischemic attack) compared to asymptomatic disease (no recent stroke). Plaques from symptomatic patients were characterized by a distinct subset of CD4+ T cells and by T cells that were activated and differentiated. Moreover, some T cell subsets in these plaques presented markers of T cell exhaustion. Additionally, macrophages from these plaques contained alternatively activated phenotypes, including subsets associated with plaque vulnerability. In plaques from asymptomatic patients, T cells and macrophages were activated and displayed evidence of interleukin-1β signaling. The identification of specific features of innate and adaptive immune cells in plaques that are associated with cerebrovascular events may enable the design of more precisely tailored cardiovascular immunotherapies.
Figures














Comment in
-
Distinct immune microenvironments in atherosclerotic plaques.Nat Rev Cardiol. 2020 Jan;17(1):7. doi: 10.1038/s41569-019-0304-0. Nat Rev Cardiol. 2020. PMID: 31654032 No abstract available.
-
CITE-Seq Hits Vascular Medicine.Clin Chem. 2020 Jun 1;66(6):751-753. doi: 10.1093/clinchem/hvaa016. Clin Chem. 2020. PMID: 32091084 Free PMC article. No abstract available.
References
-
- Libby P. Superficial erosion and the precision management of acute coronary syndromes: not one-size-fits-all. European heart journal 38, 801–803 (2017). - PubMed
-
- Moreno PR, et al. Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture. Circulation 90, 775–778 (1994). - PubMed
-
- Jawien J. The role of an experimental model of atherosclerosis: apoE-knockout mice in developing new drugs against atherogenesis. Current pharmaceutical biotechnology 13, 2435–2439 (2012). - PubMed
-
- van der Heiden K, Hoogendoorn A, Daemen MJ & Gijsen FJ Animal models for plaque rupture: a biomechanical assessment. Thrombosis and haemostasis 115(2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

