Extracting medications and associated adverse drug events using a natural language processing system combining knowledge base and deep learning
- PMID: 31591641
- PMCID: PMC7489056
- DOI: 10.1093/jamia/ocz141
Extracting medications and associated adverse drug events using a natural language processing system combining knowledge base and deep learning
Abstract
Objective: Detecting adverse drug events (ADEs) and medications related information in clinical notes is important for both hospital medical care and medical research. We describe our clinical natural language processing (NLP) system to automatically extract medical concepts and relations related to ADEs and medications from clinical narratives. This work was part of the 2018 National NLP Clinical Challenges Shared Task and Workshop on Adverse Drug Events and Medication Extraction.
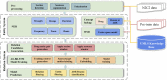
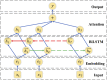
Materials and methods: The authors developed a hybrid clinical NLP system that employs a knowledge-based general clinical NLP system for medical concepts extraction, and a task-specific deep learning system for relations identification using attention-based bidirectional long short-term memory networks.
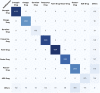
Results: The systems were evaluated as part of the 2018 National NLP Clinical Challenges challenge, and our attention-based bidirectional long short-term memory networks based system obtained an F-measure of 0.9442 for relations identification task, ranking fifth at the challenge, and had <2% difference from the best system. Error analysis was also conducted targeting at figuring out the root causes and possible approaches for improvement.
Conclusions: We demonstrate the generic approaches and the practice of connecting general purposed clinical NLP system to task-specific requirements with deep learning methods. Our results indicate that a well-designed hybrid NLP system is capable of ADE and medication-related information extraction, which can be used in real-world applications to support ADE-related researches and medical decisions.
Keywords: LSTM; UMLS; adverse drug events; attention; clinical natural language processing.
© The Author(s) 2019. Published by Oxford University Press on behalf of the American Medical Informatics Association. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

Similar articles
-
A study of deep learning approaches for medication and adverse drug event extraction from clinical text.J Am Med Inform Assoc. 2020 Jan 1;27(1):13-21. doi: 10.1093/jamia/ocz063. J Am Med Inform Assoc. 2020. PMID: 31135882 Free PMC article.
-
Clinical trial cohort selection based on multi-level rule-based natural language processing system.J Am Med Inform Assoc. 2019 Nov 1;26(11):1218-1226. doi: 10.1093/jamia/ocz109. J Am Med Inform Assoc. 2019. PMID: 31300825 Free PMC article.
-
Ensemble method-based extraction of medication and related information from clinical texts.J Am Med Inform Assoc. 2020 Jan 1;27(1):31-38. doi: 10.1093/jamia/ocz100. J Am Med Inform Assoc. 2020. PMID: 31282932 Free PMC article.
-
Overview of the First Natural Language Processing Challenge for Extracting Medication, Indication, and Adverse Drug Events from Electronic Health Record Notes (MADE 1.0).Drug Saf. 2019 Jan;42(1):99-111. doi: 10.1007/s40264-018-0762-z. Drug Saf. 2019. PMID: 30649735 Free PMC article. Review.
-
Leveraging Natural Language Processing and Machine Learning Methods for Adverse Drug Event Detection in Electronic Health/Medical Records: A Scoping Review.Drug Saf. 2025 Apr;48(4):321-337. doi: 10.1007/s40264-024-01505-6. Epub 2025 Jan 9. Drug Saf. 2025. PMID: 39786481 Free PMC article.
Cited by
-
Extracting COVID-19 diagnoses and symptoms from clinical text: A new annotated corpus and neural event extraction framework.J Biomed Inform. 2021 May;117:103761. doi: 10.1016/j.jbi.2021.103761. Epub 2021 Mar 26. J Biomed Inform. 2021. PMID: 33781918 Free PMC article.
-
Extraction of Information Related to Drug Safety Surveillance From Electronic Health Record Notes: Joint Modeling of Entities and Relations Using Knowledge-Aware Neural Attentive Models.JMIR Med Inform. 2020 Jul 10;8(7):e18417. doi: 10.2196/18417. JMIR Med Inform. 2020. PMID: 32459650 Free PMC article.
-
Clinical concept normalization with a hybrid natural language processing system combining multilevel matching and machine learning ranking.J Am Med Inform Assoc. 2020 Oct 1;27(10):1576-1584. doi: 10.1093/jamia/ocaa155. J Am Med Inform Assoc. 2020. PMID: 33029642 Free PMC article.
-
Adverse drug event detection using natural language processing: A scoping review of supervised learning methods.PLoS One. 2023 Jan 3;18(1):e0279842. doi: 10.1371/journal.pone.0279842. eCollection 2023. PLoS One. 2023. PMID: 36595517 Free PMC article.
-
Finding Potential Adverse Events in the Unstructured Text of Electronic Health Care Records: Development of the Shakespeare Method.JMIRx Med. 2021 Aug 11;2(3):e27017. doi: 10.2196/27017. JMIRx Med. 2021. PMID: 37725533 Free PMC article.
References
-
- Institute of Medicine Committee on Quality of Health Care in America. To Err Is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000. - PubMed
-
- Classen DC, Pestotnik SL, Evans RS, et al. Adverse drug events in hospitalized patients excess length of stay, extra costs, and attributable mortality. JAMA 1997; 277 (4): 301–6. - PubMed
-
- Fanikos J, Cina JL, Baroletti S, et al. Adverse drug events in hospitalized cardiac patients. Am J Cardiol 2007; 100 (9): 1465–9. - PubMed
-
- Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA 1997; 277 (4): 307–11. - PubMed
-
- Rommers MK, Teepe-Twiss IM, Guchelaar H-J.. Preventing adverse drug events in hospital practice: an overview. Pharmacoepidemiol Drug Saf 2007; 16 (10): 1129–35. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

