Botulinum toxin type A in the treatment of lower limb spasticity in children with cerebral palsy
- PMID: 31591703
- PMCID: PMC6779591
- DOI: 10.1002/14651858.CD001408.pub2
Botulinum toxin type A in the treatment of lower limb spasticity in children with cerebral palsy
Abstract
Background: Cerebral palsy (CP) is the most common cause of physical disabilities in children in high-income countries. Spasticity is the most common motor disturbance in CP. Botulinum toxin type A (BoNT-A) is considered the first-line treatment for focal spasticity in people with CP.
Objectives: To evaluate the effectiveness and safety of BoNT-A compared to other treatments used in the management of lower limb spasticity in children with CP.
Search methods: We searched CENTRAL, PubMed, four other databases, and two trial registers in October 2018. We also searched the reference lists of relevant studies and reviews and contacted experts in the field. We did not apply any date or language restrictions.
Selection criteria: Randomised controlled trials of children with CP, aged between birth and 19 years, treated with BoNT-A injections in the lower limb muscles compared to other interventions. The primary outcomes were gait analysis and function. The secondary outcomes were joint range of motion, quality of life, satisfaction, spasticity, and adverse events.
Data collection and analysis: Two review authors independently selected studies, extracted data, assessed risk of bias, and rated the quality of the evidence using GRADE. A third review author arbitrated in case of disagreements. We conducted meta-analyses of available data whenever possible, analysing dichotomous data with risk ratios (RR), and continuous data with mean differences (MD) or standardised mean differences (SMD), with 95% confidence intervals (CI). We considered a 5% significance level for all analyses.Whenever possible, we analysed outcomes at the time points at which they were assessed: short term (2 to 8 weeks); medium term (12 to 16 weeks); and long term (> 24 weeks).
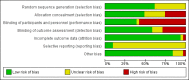
Main results: We included 31 randomised controlled trials assessing 1508 participants. Most studies included ambulatory patients with more than one motor type of CP, and with a mean age of between three and seven years. There was a slight predominance of males.Studies compared BoNT-A in the lower limb muscles to usual care or physiotherapy (14 studies), placebo or sham (12 studies), serial casting (4 studies), or orthoses (1 study).We rated studies as at high or unclear risk of bias mainly due to random sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment.BoNT-A versus usual care or physiotherapyBoNT-A might improve overall gait scores at medium-term follow-up (MD 2.80, 95% CI 1.55 to 4.05; 1 study, 40 children; very low-quality evidence) and is moderately effective at improving function at short-term (SMD 0.59, 95% CI 0.23 to 0.95; 2 studies, 123 children) and medium-term (SMD 1.04, 95% CI 0.16 to 1.91; 4 studies, 191 children) follow-up (all very low-quality evidence).BoNT-A improves ankle range of motion, satisfaction, and ankle plantarflexors spasticity at one or more time points (very low-quality evidence).The proportion of adverse events in the BoNT-A group was 0.37 (95% CI 0.08 to 0.66; I2 = 95%; very low-quality evidence). No adverse events were reported in the control group.BoNT-A versus placebo or shamBoNT-A improves overall gait scores at short-term (RR 1.66, 95% CI 1.16 to 2.37, P = 0.006; 4 studies, 261 assessments) and medium-term (RR 1.90, 95% CI 1.32 to 2.74, P < 0.001; 3 studies, 248 assessments) follow-up, and may improve peak ankle dorsiflexion in stance (MD 15.90 degrees, 95% CI 4.87 to 26.93, P = 0.005; 1 study, 19 children) and in swing (MD 10.20 degrees, 95% CI 4.01 to 16.39, P = 0.001; 1 study, 19 children) at short-term follow-up (all moderate-quality evidence).BoNT-A is not more effective than placebo or sham at improving function at short-term (SMD 0.24, 95% CI -0.35 to 0.83, P = 0.42; 4 studies, 305 children) or long-term (SMD -0.07, 95% CI -0.48 to 0.35, P = 0.76; 2 studies, 91 children) follow-up, but has a small positive effect at medium-term follow-up (SMD 0.28, 95% CI 0.06 to 0.49, P = 0.01; 5 studies, 327 children) (all moderate-quality evidence).BoNT-A improves passive ankle range of motion, satisfaction, and ankle plantarflexors spasticity at one or more time points (moderate-quality evidence).There was no difference between groups in the rate of adverse events at short-term follow-up (RR 1.29, 95% CI 0.87 to 1.93, P = 0.21; 12 studies, 918 children; moderate-quality evidence).BoNT-A versus serial castingThere was no difference between groups for overall gait scores at short-term (MD 0.00, 95% CI -1.66 to 1.66); medium-term (MD 0.65, 95% CI -1.21 to 2.51); or long-term (MD 0.46, 95% CI -1.33 to 2.25) follow-up in one study with 18 children (moderate-quality evidence).BoNT-A improved instrumented gait analysis only in terms of ankle dorsiflexion at initial contact (MD 6.59 degrees, 95% CI 1.39 to 11.78, P = 0.01; 2 studies, 47 children). There was no difference between groups for peak ankle dorsiflexion in stance and swing, and gait speed at any time point (moderate- and low-quality evidence).BoNT-A is not more effective than serial casting at improving function, ankle range of motion, and spasticity at any time point (moderate- and low-quality evidence).BoNT-A is not associated with a higher risk of adverse events than serial casting (RR 0.59, 95% CI 0.03 to 11.03; 3 studies, 64 children; low-quality evidence).BoNT-A versus orthosesThere was no difference between groups for function at medium-term follow-up (MD 11.14, 95% CI -0.05 to 22.33; 1 study, 43 children), but BoNT-A is more effective than orthoses at improving hip range of motion and hip adductors spasticity (all very low-quality evidence).
Authors' conclusions: The quality of the evidence was low or very low for most of the outcomes analysed. We found limited evidence that BoNT-A is more effective than placebo or a non-placebo control at improving gait, joint range of motion, satisfaction, and lower limb spasticity in children with CP, whereas the results for function were contradictory. The rate of adverse events with BoNT-A is similar to placebo. BoNT-A is not more effective than ankle serial casting to treat ankle contractures for any of the assessed outcomes, but is more effective than orthotics at improving range of motion and spasticity.
Conflict of interest statement
Francesco C Blumetti ‐ none known. João Carlos Belloti ‐ none known. Marcel Jun Tamaoki ‐ none known. José A Pinto ‐ none known.
Figures



































































Update of
References
References to studies included in this review
Ackman 2005 {published and unpublished data}
-
- Ackman JD, Russman BS, Thomas SS, Buckon CE, Sussman MD, Masso P, et al. Comparing botulinum toxin A with casting for treatment of dynamic equinus in children with cerebral palsy. Developmental Medicine & Child Neurology 2005;47(9):620‐7. [PUBMED: 16138670] - PubMed
-
- Thomas SS. Systematic review [personal communication]. Email to: FC Blumetti 2 February 2016.
Baker 2002 {published data only}
-
- Baker R, Jasinski M, Maciag‐Tymecka I, Michalowska‐Mrozek J, Bonikowski M, Carr L, et al. Botulinum toxin treatment of spasticity in diplegic cerebral palsy: a randomized, double‐blind, placebo‐controlled, dose‐ranging study. Developmental Medicine & Child Neurology 2002;44(10):666‐75. [DOI: 10.1017/s0012162201002730; PUBMED: 12418791] - DOI - PubMed
Barwood 2000 {published data only}
Bjornson 2007 {published data only}
Boyd 2001 {published data only}
-
- Boyd RN, Dobson F, Parrott J, Love S, Oates J, Larson A, et al. The effect of botulinum toxin type A and a variable hip abduction orthosis on gross motor function: a randomized controlled trial. European Journal of Neurology 2001;8 Suppl 5:109‐19. [PUBMED: 11851739] - PubMed
-
- Graham HK, Boyd R, Carlin JB, Dobson F, Lowe K, Nattrass G, et al. Does botulinum toxin A combined with bracing prevent hip displacement in children with cerebral palsy and "hips at risk"? A randomized, controlled trial. Journal of Bone & Joint Surgery 2008;90(1):23‐33. [DOI: 10.2106/JBJS.F.01416; PUBMED: 18171954] - DOI - PubMed
Çağlar 2019 {published data only}
Chaturvedi 2013 {published data only}
-
- Chaturvedi SK, Rai Y, Chourasia A, Goel P, Paliwal VK, Garg RK, et al. Comparative assessment of therapeutic response to physiotherapy with or without botulinum toxin injection using diffusion tensor tractography and clinical scores in term diplegic cerebral palsy children. Brain & Development 2013;35(7):647‐53. [DOI: 10.1016/j.braindev.2012.10.012; PUBMED: 23165172] - DOI - PubMed
Copeland 2014 {published data only}
-
- Copeland L, Edwards P, Thorley M, Donaghey S, Gascoigne‐Pees L, Kentish M, et al. Botulinum toxin A for nonambulatory children with cerebral palsy: a double blind randomized controlled trial. Journal of Pediatrics 2014;165(1):140‐6.e4. [DOI: 10.1016/j.jpeds.2014.01.050; PUBMED: 24630348] - DOI - PubMed
Corry 1998 {published and unpublished data}
-
- Corry IS, Cosgrove AP, Duffy CM, McNeill S, Taylor TC, Graham HK. Botulinum toxin A compared with stretching casts in the treatment of spastic equinus: a randomised prospective trial. Journal of Pediatric Orthopaedics 1998;18(3):304‐11. [PUBMED: 9600553] - PubMed
Delgado 2016 {published data only}
-
- Tilton A, Russman B, Aydin R, Dincer U, Escobar RG, Kutlay S, et al. AbobotulinumtoxinA (Dysport®) improves function according to goal attainment in children with dynamic equinus due to cerebral palsy. Journal of Child Neurology 2017;32(5):482‐7. [DOI: 10.1177/0883073816686910; PMC5405835; PUBMED: 28068857] - DOI - PMC - PubMed
El‐Etribi 2004 {published data only}
-
- El‐Etribi MA, Salem ME, El‐Shakankiry HM, El‐Kahky AM, El‐Mahboub SM. The effect of botulinum toxin type‐A injection on spasticity, range of motion and gait patterns in children with spastic diplegic cerebral palsy: an Egyptian study. International Journal of Rehabilitation Research 2004;27(4):275‐81. [PUBMED: 15572990] - PubMed
Flett 1999 {published data only}
Hazneci 2006 {published data only}
-
- Hazneci B, Tan AK, Guncikan MN, Dincer K, Kalyon TA. Comparison of the efficacies of botulinum toxin A and Johnstone pressure splints against hip adductor spasticity among patients with cerebral palsy: a randomized trial. Military Medicine 2006;171(7):653‐6. [DOI: 10.7205/milmed.171.7.653; PUBMED: 16895135] - DOI - PubMed
Ibrahim 2007 {published data only}
-
- Ibrahim AI, Hawamdeh ZM, Al‐Qudah AA. Functional outcome of botulinum toxin injection of gastrocnemius and adductors in spastic hemiplegic cerebral palsied children. Europa Medicophysica 2007;43(1):13‐20. [PUBMED: 17021584] - PubMed
Jozwiak 2007 {published data only}
-
- Jozwiak M, Harasymczuk P, Ciemniewska‐Gorzela K. The use of botulinum toxin in the treatment of spastic hip joint instability in children with cerebral palsy. Chirurgia Narzadow Ruchu i Ortopedia Polska 2007;72(3):205‐9. [PUBMED: 17941584] - PubMed
Kanovsky 2004 {published data only}
-
- Kanovsky P, Bares M, Severa S, Benetin J, Kraus J, Richardson A, et al. Functional benefit of botulinum toxin (Dysport®) in the treatment of dynamic equinus cerebral palsy spasticity: a prospective, multicentre, double‐blind, placebo‐controlled study. Ceska a Slovenska Neurologie a Neurochirurgie 2004;67:16‐23.
Kay 2004 {published data only}
-
- Kay RM, Rethlefsen SA, Fern‐Buneo A, Wren TA, Skaggs DL. Botulinum toxin as an adjunct to serial casting treatment in children with cerebral palsy. Journal of Bone and Joint Surgery 2004;86‐a:2377‐84. - PubMed
Koman 1994 {published data only}
-
- Koman LA, Mooney JF, Smith BP, Goodman A, Mulvaney T. Management of spasticity in cerebral palsy with botulinum‐A toxin: report of a preliminary, randomized, double‐blind trial. Journal of Pediatric Orthopaedics 1994;14(3):299‐303. - PubMed
Koman 2000 {published data only}
-
- Koman LA, Mooney JF 3rd, Smith BP, Walker F, Leon JM. Botulinum toxin type A neuromuscular blockade in the treatment of lower extremity spasticity in cerebral palsy: a randomized, double‐blind, placebo‐controlled trial. BOTOX Study Group. Journal of Pediatric Orthopaedics 2000;20:108‐15. - PubMed
Love 2001 {published data only}
-
- Love SC, Valentine JP, Blair EM, Price CJ, Cole JH, Chauvel PJ. The effect of botulinum toxin type A on the functional ability of the child with spastic hemiplegia a randomized controlled trial. European Journal of Neurology 2001;8 Suppl 5:50‐8. [PUBMED: 11851734] - PubMed
Mall 2006 {published data only}
-
- Mall V, Heinen F, Siebel A, Bertram C, Hafkemeyer U, Wissel J, et al. Treatment of adductor spasticity with BTX‐A in children with CP: a randomized, double‐blind, placebo‐controlled study. Developmental Medicine & Child Neurology 2006;48(1):10‐3. [DOI: 10.1017/S0012162206000041; PUBMED: 16359588] - DOI - PubMed
Moore 2008 {published data only}
Navarrete 2010 {published data only}
-
- Navarrete ÁA, Peters D, Ruz S. Functional outcome of multilevel botulinum toxin infiltration of the lower limbs and comprehensive therapy in children with spastic cerebral palsy [Resultado funcional de infiltración de toxina botulínica multinivel de las extremidades inferiores y terapia integral en niños con parálisis cerebral espástica]. Rehabilitación 2010;44(3):236‐43. [DOI: 10.1016/j.rh.2009.11.010] - DOI
Reddihough 2002 {published data only}
Scholtes 2006 {published data only}
-
- Scholtes VA, Dallmeijer AJ, Knol DL, Speth LA, Maathuis CG, Jongerius PH, et al. The combined effect of lower‐limb multilevel botulinum toxin type A and comprehensive rehabilitation on mobility in children with cerebral palsy: a randomized clinical trial. Archives of Physical Medicine and Rehabilitation 2006;87(12):1551‐8. [DOI: 10.1016/j.apmr.2006.08.342; PUBMED: 17141633] - DOI - PubMed
Steenbeek 2005 {published data only}
-
- Steenbeek D, Meester‐Delver A, Becher JG, Lankhorst GJ. The effect of botulinum toxin type A treatment of the lower extremity on the level of functional abilities in children with cerebral palsy: evaluation with goal attainment scaling. Clinical Rehabilitation 2005;19(3):274‐82. [DOI: 10.1191/0269215505cr859oa; PUBMED: 15859528] - DOI - PubMed
Sutherland 1999 {published data only}
Tedroff 2010 {published data only}
Ubhi 2000 {published data only}
Xu 2006 {published data only}
-
- Xu KS, Yan TB, Mai JN. Effects of botulinum toxin guided by electric stimulation on spasticity in ankle plantar flexor of children with cerebral palsy: a randomized trial [Diàn cìjī dìngwèi yǐndǎo ròu dú dúsù zhìliáo nǎoxìngtānhuàn huànér huái zhí qū jī qún jìngluán de duìzhào yánjiū]. Zhonghua Er Ke Za Zhi [Chinese Journal of Pediatrics] 2006;44(12):913‐7. [PUBMED: 17254459] - PubMed
Zhu 2016 {published data only}
-
- Zhu DN, Wang MM, Wang J, Zhang W, Li HZ, Yang P, et al. Effect of botulinum toxin A injection in the treatment of gastrocnemius spasticity in children aged 9‐36 months with cerebral palsy: a prospective study [Ròu dú gǎnjùn dúsù A zhùshè yè zhìliáo 9‐36 gè yuè nǎotān huàn ér féichángjī jìngluán de qiánzhān xìng yánjiū]. Zhongguo Dang Dai Er Ke Za Zhi [Chinese Journal of Contemporary Pediatrics] 2016;18(2):123‐9. [DOI: 10.7499/j.issn.1008-8830.2016.02.006; PUBMED: 26903058] - DOI - PMC - PubMed
References to studies excluded from this review
Ackman 1998 {published data only}
-
- Ackman J, Abu‐Faraj Z, Chambers C, Philips B, Davids J. Botulinum toxin treatment of dynamic deformities in an ambulatory spastic cerebral palsy population: a multi‐center study. Gait & Posture 1998;7(2):167. [DOI: 10.1016/S0966-6362(98)90245-4] - DOI
Amirsalari 2011 {published data only}
-
- Amirsalari S, Dalvand H, Dehghan L, Feizy A, Hosseini SAA, Shamsodini A. The efficacy of botulinum toxin type A injection in the hamstring and calf muscles with and without serial foot casting in gait improvement in children with cerebral palsy. Tehran University Medical Journal 2011; Vol. 69, issue 8:509‐17. [tumj.tums.ac.ir/article‐1‐208‐en.html]
Ayllón 2003 {published data only}
-
- Ayllón C, Prieto F, Tsuruoka M, Cermignani E, Guerrini de Larrañaga N. Botulin toxin A ‐ treatment of spastic cerebral palsy. Pediatric Research 2003;53:873. [DOI: 10.1203/00006450-200305000-00056] - DOI
Barber 2013 {published data only}
-
- Barber L, Hastings‐Ison T, Baker R, Kerr Graham H, Barrett R, Lichtwark G. The effects of botulinum toxin injection frequency on calf muscle growth in young children with spastic cerebral palsy: a 12‐month prospective study. Journal of Children's Orthopaedics 2013;7(5):425‐33. [DOI: 10.1007/s11832-013-0503-x; PMC3838523; PUBMED: 24432106] - DOI - PMC - PubMed
Bostock 2009 {published data only}
-
- Bostock S, Gibson S, Russo R, Flett P. Who needs serial casting? The profile of children with cerebral palsy who did not require serial casting following botulinum toxin (A) treatment for dynamic equinus. Developmental Medicine & Child Neurology 2009;51(Suppl 2):9. [DOI: 10.1111/j.1469-8749.2008.03237.x] - DOI
Bottos 2003 {published data only}
-
- Bottos M, Benedetti MG, Salucci P, Gasparroni V, Giannini S. Botulinum toxin with and without casting in ambulant children with spastic diplegia: a clinical and functional assessment. Developmental Medicine & Child Neurology 2003;45(11):758‐62. [DOI: 10.1017/s0012162203001403; PUBMED: 14580131] - DOI - PubMed
Carraro 2016 {published data only}
-
- Carraro E, Trevisi E, Martinuzzi A. Safety profile of incobotulinum toxin A (Xeomin®) in gastrocnemious muscles injections in children with cerebral palsy: randomized double‐blind clinical trial. European Journal of Paediatric Neurology 2016;20(4):532‐7. [DOI: 10.1016/j.ejpn.2016.04.008] - DOI - PubMed
Chang 2017 {published data only}
-
- Chang HJ, Hong BY, Lee SJ, Lee S, Park JH, Kwon JY. Efficacy and safety of letibotulinum toxin A for the treatment of dynamic equinus foot deformity in children with cerebral palsy: a randomized controlled trial. Toxins 2017;9(8):250. [DOI: 10.3390/toxins9080252; PMC5577586; PUBMED: 28820439] - DOI - PMC - PubMed
Dabrowski 2018 {published data only}
Dai 2017 {published data only}
-
- Dai AI, Demiryürek AT. Serial casting as an adjunct to botulinum toxin type A treatment in children with cerebral palsy and spastic paraparesis with scissoring of the lower extremities. Journal of Child Neurology 2017; Vol. 32, issue 7:671‐5. [DOI: 10.1177/0883073817701526; PUBMED: 28393669] - DOI - PubMed
Dalvand 2012 {published data only}
-
- Dalvand H, Dehghan L, Feizi A, Amir Salari S, Hosseini SA, Shamsoddini A. The effect of foot serial casting along with botulinum toxin type‐A injection on spasticity in children with cerebral palsy. Journal of Kerman University of Medical Sciences 2012;19(6):562‐73. [www.sid.ir/en/journal/ViewPaper.aspx?id=288007]
Desloovere 2001 {published data only}
-
- Desloovere K, Molenaers G, Jonkers I, Cat J, Borre L, Nijs J, et al. A randomized study of combined botulinum toxin type A and casting in the ambulant child with cerebral palsy using objective outcome measures. European Journal of Neurology 2001;8 Suppl 5:75‐87. [PUBMED: 11851736] - PubMed
Detrembleur 2002 {published data only}
Dimitrijević 2007 {published data only}
-
- Dimitrijević L, Stanković I, Živković V, Mikov A, Čolović H, Janković I. Botulinum toxin type A for the treatment of spasticity in children with cerebral palsy [Primena botulinskog toksina tip A u lečenju spasticiteta kod dece sa cerebralnom paralizom]. Vojnosanitetski Pregled 2007;64(8):513‐8. [DOI: 10.2298/VSP0708513D; PUBMED: 17874717] - DOI - PubMed
Dincer 2008 {published data only}
-
- Dincer Ü, Cakar E, Kiralp MZ, Dursun H. Comparison of the effectiveness of physiotherapy and ankle foot orthosis after botulinum toxin injection in diplegic cerebral palsy patients [Diplejik serebral palsili hastalarda botulinum toksin uygulamasi sonrasinda fizyoterapi ve alt ekstremite ortezinin etkinliginin karsilastirilmasi]. Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi 2008;54(2):41‐5. [www.ftrdergisi.com/uploads/sayilar/235/buyuk/41‐451.pdf]
Flemban 2018 {published data only}
-
- Flemban A, Elsayed W. Effect of combined rehabilitation program with botulinum toxin type A injections on gross motor function scores in children with spastic cerebral palsy. Journal of Physical Therapy Science 2018; Vol. 30, issue 7:902‐5. [DOI: 10.1589/jpts.30.902; PMC6047965; PUBMED: 30034093] - DOI - PMC - PubMed
Hansen 2011 {published data only}
Hastings‐Ison 2016 {published data only}
Hawamdeh 2007 {published data only}
-
- Hawamdeh ZM, Ibrahim AI, Al‐Qudah AA. Long‐term effect of botulinum toxin (A) in the management of calf spasticity in children with diplegic cerebral palsy. Europa Medicophysica 2007;43(3):311‐8. [PUBMED: 17268388] - PubMed
Hong 2017 {published data only}
-
- Hong BY, Chang HJ, Lee SJ, Lee S, Park JH, Kwon JY. Efficacy of repeated botulinum toxin type A injections for spastic equinus in children with cerebral palsy ‐ a secondary analysis of the randomized clinical trial. Toxins 2017; Vol. 9, issue 8:253. [DOI: 10.3390/toxins9080253; PMC5577587; PUBMED: 28825663] - DOI - PMC - PubMed
Hu 2009 {published data only}
-
- Hu GC, Chuang YC, Liu JP, Chien KL, Chen YM, Chen YF. Botulinum toxin (Dysport) treatment of the spastic gastrocnemius muscle in children with cerebral palsy: a randomized trial comparing two injection volumes. Clinical Rehabilitation 2009;23(1):64‐71. [DOI: 10.1177/0269215508097861; PUBMED: 19114438] - DOI - PubMed
Javadzadeh 2006 {published data only}
-
- Javadzadeh M, Akbari A, Zadeh‐Vakili A, Moghtaderi A, Mahvelati F. Comparing the effectiveness of different doses of botulinum toxin on improvement of walking and reducing the spasticity in spastic diplegic cerebral palsy. European Journal of Pediatrics 2006; Vol. 165, issue Suppl 1:216. [DOI: 10.1007/s00431-006-0349-z] - DOI
Jianjun 2013 {published data only}
Kang 2007 {published data only}
Kanovsky 2009 {published data only}
-
- Kanovský P, Bares M, Severa S, Richardson A, Dysport Paediatric Limb Spasticity Study Group. Long‐term efficacy and tolerability of 4‐monthly versus yearly botulinum toxin type A treatment for lower‐limb spasticity in children with cerebral palsy. Developmental Medicine & Child Neurology 2009;51(6):436‐45. [DOI: 10.1111/j.1469-8749.2008.03264.x; PUBMED: 19563586] - DOI - PubMed
Kelly 2019 {published data only}
-
- Kelly B, MacKay‐Lyons M, Berryman S, Hyndman J, Wood E. Casting protocols following BoNT‐A injections to treat spastic hypertonia of the triceps surae in children with cerebral palsy and equinus gait: a randomized controlled trial. Physical & Occupational Therapy Pediatrics 2019; Vol. 39, issue 1:77‐93. [DOI: 10.1080/01942638.2018.1471015; PUBMED: 29771161] - DOI - PubMed
Kim 2011 {published data only}
-
- Kim K, Shin HI, Kwon BS, Kim SJ, Jung IY, Bang MS. Neuronox versus BOTOX for spastic equinus gait in children with cerebral palsy: a randomized, double‐blinded, controlled multicentre clinical trial. Developmental Medicine & Child Neurology 2011;53(3):239‐44. [DOI: 10.1111/j.1469-8749.2010.03830.x; PUBMED: 21087238] - DOI - PubMed
Kurenkov 2017 {published data only}
-
- Kurenkov AL, Klochkova OA, Bursagova BI, Karimova HM, Kuzenkova LM, Mamedyarov AM, et al. Efficacy and safety of botulinum toxin type A (IncobotulinumtoxinA) in the treatment of patients with cerebral palsy [Èffektivnostʹ i bezopasnostʹ primenenija botuliničeskogo toksina tipa A (IncobotulinumtoxinA) pri lečenii pacientov s detskim cerebralʹnym paraličom]. Zhurnal Nevrologii i Psikhiatrii imeni SS Korsakova 2017; Vol. 117, issue 11:37‐44. [DOI: 10.17116/jnevro201711711137-44; PUBMED: 29265085] - DOI - PubMed
Kwon 2010 {published data only}
-
- Kwon JY, Hwang JH, Kim JS. Botulinum toxin A injection into calf muscles for treatment of spastic equinus in cerebral palsy: a controlled trial comparing sonography and electric stimulation‐guided injection techniques: a preliminary report. American Journal of Physical Medicine & Rehabilitation 2010;89(4):279‐86. [DOI: 10.1097/PHM.0b013e3181ca24ac; PUBMED: 20068435] - DOI - PubMed
Lee 2009 {published data only}
-
- Lee JH, Sung IY, Yoo JY, Park EH, Park SR. Effects of different dilutions of botulinum toxin type A treatment for children with cerebral palsy with spastic ankle plantarflexor: a randomized controlled trial. Journal of Rehabilitation Medicine 2009;41(9):740‐5. [DOI: 10.2340/16501977-0418; PUBMED: 19774308] - DOI - PubMed
Lee 2011 {published data only}
-
- Lee SJ, Sung IY, Jang DH, Yi JH, Lee JH, Ryu JS. The effect and complication of botulinum toxin type A injection with serial casting for the treatment of spastic equinus foot. Annals of Rehabilitation Medicine 2011;35(3):344‐53. [DOI: 10.5535/arm.2011.35.3.344; PMC3309222; PUBMED: 22506143] - DOI - PMC - PubMed
Love 2009 {published data only}
-
- Love S, Blair E, Valentine J, Cole J. Should we be using BoNT‐A earlier?. Developmental Medicine & Child Neurology 2009;51(Suppl 2):8. [DOI: 10.1111/j.1469-8749.2008.03237.x] - DOI
Mohamed 2001 {published data only}
Newman 2007 {published data only}
-
- Newman CJ, Kennedy A, Walsh M, O'Brien T, Lynch B, Hensey O. A pilot study of delayed versus immediate serial casting after botulinum toxin injection for partially reducible spastic equinus. Journal of Pediatric Orthopaedics 2007;27(8):882‐5. [DOI: 10.1097/BPO.0b013e31815b4d7d; PUBMED: 18209608] - DOI - PubMed
Niu 2014 {published data only}
-
- Niu GH, Zhang XL, Zhu DN, Cai ZJ, Li SS, Zhang W. Therapeutic effects of different doses of botulinum toxin A injection on tiptoe deformation in children with cerebral palsy [Bùtóng jìliàng A xíng ròu dú dúsù zhùshè zhìliáo nǎo xìng tānhuàn jiān zú de liáoxiào duìbǐ yánjiū]. Zhongguo Dang Dai Er Ke Za Zhi [Chinese Journal of Contemporary Pediatrics] 2014;16(7):720‐4. [DOI: 10.7499/j.issn.1008-8830.2014.07.013; PUBMED: 25008880] - DOI - PubMed
Park 2010 {published data only}
-
- Park ES, Rha DW, Yoo JK, Kim SM, Chang WH, Song SH. Short‐term effects of combined serial casting and botulinum toxin injection for spastic equinus in ambulatory children with cerebral palsy. Yonsei Medical Journal 2010;51(4):579‐84. [DOI: 10.3349/ymj.2010.51.4.579; PMC2880273; PUBMED: 20499426] - DOI - PMC - PubMed
Peeters 2018 {published data only}
-
- Peeters N, Hanssen B, Cenni F, Schless SH, Beukelaer N, Degelaen M, et al. O 019 ‐ do botulinum toxin‐A and lower leg casting alter calf muscle and tendon lengths in children with spastic cerebral palsy?. Gait & Posture 2018; Vol. 65 Suppl 1:36‐8. [DOI: 10.1016/j.gaitpost.2018.06.037] - DOI
Picelli 2017 {published data only}
-
- Picelli A, Marchina E, Gajofatto F, Pontillo A, Vangelista A, Filippini R, et al. Sonographic and clinical effects of botulinum toxin type A combined with extracorporeal shock wave therapy on spastic muscles of children with cerebral palsy. Developmental Neurorehabilitation 2017; Vol. 20, issue 3:160‐4. [DOI: 10.3109/17518423.2015.1105320; PUBMED: 26890193] - DOI - PubMed
Polak 2002 {published data only}
-
- Polak F, Morton R, Ward C, Wallace WA, Doderlein L, Siebel A. Double‐blind comparison study of two doses of botulinum toxin A injected into calf muscles in children with hemiplegic cerebral palsy. Developmental Medicine & Child Neurology 2002;44(8):551‐5. [DOI: 10.1017/s0012162201002547; PUBMED: 12206622] - DOI - PubMed
Richman 1996 {published data only}
-
- Richman DA, Gaebler‐Spira DJ. The use of botulinum A toxin to improve quality of life of children with cerebral palsy in a long term health care facility. Archives of Physical Medicine and Rehabilitation 1996;77(9):923. [DOI: 10.1016/S0003-9993(96)90288-9] - DOI
Robertshaw 2005 {published data only}
Sätilä 2005 {published data only}
-
- Sätilä H, Iisalo T, Pietikäinen T, Seppänen RL, Salo M, Koivikko M, et al. Botulinum toxin treatment of spastic equinus in cerebral palsy: a randomized trial comparing two injection sites. American Journal of Physical Medicine & Rehabilitation 2005;84(5):355‐65; quiz 366‐7, 392. [PUBMED: 15829782] - PubMed
Sätilä 2006 {published data only}
Sätilä 2008 {published data only}
-
- Sätilä H, Pietikäinen T, Iisalo T, Lehtonen‐Räty P, Salo M, Haataja R, et al. Botulinum toxin type A injections into the calf muscles for treatment of spastic equinus in cerebral palsy: a randomized trial comparing single and multiple injection sites. American Journal of Physical Medicine & Rehabilitation 2008;87(5):386‐94. [DOI: 10.1097/PHM.0b013e31816ddaa9; PUBMED: 18427220] - DOI - PubMed
Schasfoort 2017 {published data only}
-
- Schasfoort FC, Pangalila RF, Dallmeijer AJ, Catsman CE, Sneekes EM, Eline E, et al. Evaluation of the added value of botulinum toxin treatment and intensive physiotherapy to improve body function and structure in children with spastic cerebral palsy. Developmental Medicine & Child Neurology 2017; Vol. 59, issue Suppl 2:21. [DOI: 10.1111/dmcn.13455; oral presentation 48] - DOI
Schasfoort 2018 {published data only}
-
- Schasfoort F, Pangalila R, Sneekes EM, Catsman C, Becher J, Horemans H, et al. Intramuscular botulinum toxin prior to comprehensive rehabilitation has no added value for improving motor impairments, gait kinematics and goal attainment in walking children with spastic cerebral palsy. Journal of Rehabilitation Medicine 2018; Vol. 50, issue 8:732‐42. [DOI: 10.2340/16501977-2369; PUBMED: 30080235] - DOI - PubMed
Thomas 2012 {published data only}
-
- Thomas R, Johnston LM, Boyd R, Sakzewski L, Case V, Kentish M. GR IN: randomised trial of group versus individual models of physiotherapy for ambulant children with cerebral palsy following lower limb botulinum toxin‐A. Developmental Medicine & Child Neurology 2012;54(Suppl 5):46‐7. [DOI: 10.1111/j.1469-8749.2012.04289.x; F3.F2] - DOI
Thorley 2012 {published data only}
-
- Thorley M, Donaghey S, Edwards P, Copeland L, Kentish M, McLennan K, et al. Evaluation of the effects of botulinum toxin A injections when used to improve ease of care and comfort in children with cerebral palsy whom are non‐ambulant: a double blind randomized controlled trial. BMC Pediatrics 2012;12:120. [DOI: 10.1186/1471-2431-12-120; PMC3472230; PUBMED: 22873758] - DOI - PMC - PubMed
Van Campenhout 2013 {published data only}
-
- Campenhout A, Verhaegen A, Pans S, Molenaers G. Botulinum toxin type A injections in the psoas muscle of children with cerebral palsy: muscle atrophy after motor end plate‐targeted injections. Research in Developmental Disabilities 2013;34(3):1052‐8. [DOI: 10.1016/j.ridd.2012.11.016; PUBMED: 23295965] - DOI - PubMed
Van Campenhout 2015 {published data only}
-
- Campenhout A, Bar‐On L, Desloovere K, Huenaerts C, Molenaers G. Motor endplate‐targeted botulinum toxin injections of the gracilis muscle in children with cerebral palsy. Developmental Medicine & Child Neurology 2015; Vol. 57, issue 5:476‐83. [DOI: 10.1111/dmcn.12667; PUBMED: 25557985] - DOI - PubMed
Wang 2007 {published data only}
-
- Wang YJ, Yan GY, Gao BQ. Management of spastic cerebral palsy with botulinum‐A toxin: relationship between dosage and efficacy. Zhongguo Dang Dai Er Ke Za Zhi [Chinese journal of contemporary pediatrics] 2007;9(3):247‐8. [PUBMED: 17582266] - PubMed
Wang 2008 {published data only}
Williams 2013 {published data only}
-
- Williams SA, Elliott C, Valentine J, Gubbay A, Shipman P, Reid S. Combining strength training and botulinum neurotoxin intervention in children with cerebral palsy: the impact on muscle morphology and strength. Disability and Rehabilitation 2013;35(7):596‐605. [DOI: 10.3109/09638288.2012.711898; PUBMED: 22928803] - DOI - PubMed
Wissel 1999 {published data only}
-
- Wissel J, Heinen F, Schenkel A, Doll B, Ebersbach G, Müller J, et al. Botulinum toxin A in the management of spastic gait disorders in children and young adults with cerebral palsy: a randomized, double‐blind study of "high‐dose" versus "low‐dose" treatment. Neuropediatrics 1999;30(3):120‐4. [DOI: 10.1055/s-2007-973475; PUBMED: 10480205] - DOI - PubMed
Wong 2005 {published data only}
-
- Wong AM, Pei YC, Lui TN, Chen CL, Wang CM, Chung CY. Comparison between botulinum toxin type A injection and selective posterior rhizotomy in improving gait performance in children with cerebral palsy. Journal of Neurosurgery 2005;102(4 Suppl):385‐9. [DOI: 10.3171/ped.2005.102.4.0385; PUBMED: 15926389] - DOI - PubMed
Xu 2009 {published data only}
YaJie 2008 {published data only}
Zier 2008 {published data only}
Zonta 2013 {published data only}
References to studies awaiting assessment
Kim 2018 {published data only}
-
- Kim H, Meilahn J, Liu C, Chambers HG, Dimitrova R. Efficacy and safety of onabotulinumtoxinA for the treatment of pediatric lower limb spasticity: primary results (S29.007). Neurology 2018;90(15 Suppl). [ISSN: 1526‐632X; n.neurology.org/content/90/15_Supplement/S29.007]
References to ongoing studies
ACTRN12615001162505 {unpublished data only}
-
- ACTRN12615001162505. The impact of Botox on muscle structure and function in children with cerebral palsy [A RCT of the efficacy of the first intramuscular botulinum toxin type‐A treatment on muscle structure and function in children with cerebral palsy: a multi‐centre trial]. www.anzctr.org.au/ACTRN12615001162505.aspx (registered 30 october 2015).
CTRI/2015/03/005642 {unpublished data only}
-
- CTRI/2015/03/005642. Clinical trial to compare the effect of botulinum toxin vs surgical management of lower limb deformities in children with spastic cerebral palsy [Botulinum toxin versus singe event multi‐level surgery (SEMLS) in children with lower limb deformities in spastic cerebral palsy ‐ a randomised comparative trial]. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=8038 (registered 19 March 2015).
NCT02400619 {unpublished data only}
-
- NCT02400619. Shockwaves therapy and botulinum toxin for the treatment of spasticity in patients with cerebral palsy: a cross over RCT [Efficacy of radial extracorporeal shock waves compared to botulinum toxin type A in the treatment of spasticity of the lower extremities in patients with cerebral palsy: a crossover randomized clinical trial]. clinicaltrials.gov/show/NCT02400619 (registered 27 March 2015).
NCT02546999 {unpublished data only}
-
- NCT02546999. Does botulinum toxin A make walking easier in children with cerebral palsy?. clinicalTrials.gov/show/NCT02546999 (registered 11 September 2015).
TCTR20150803003 {unpublished data only}
-
- TCTR20150803003. Efficacy of botulinum toxin in pediatric spasticity: a randomized trial comparing 2‐ to 4‐ sites hamstring injection. www.clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&... (registered 3 August 2015).
Additional references
Blair 2010
Bohannon 1987
Boyd 1999
-
- Boyd RN, Graham HK. Objective measurement of clinical findings in the use of botulinum toxin type A for the management of children with cerebral palsy. European Journal of Neurology 1999;6(Suppl 4):s23–35. [DOI: 10.1111/j.1468-1331.1999.tb00031.x] - DOI
Boyd 2001a
-
- Boyd RN, Dobson F, Parrott J, Love S, Oates J, Larson A, et al. The effect of botulinum toxin type A and a variable hip abduction orthosis on gross motor function: a randomized controlled trial. European Journal of Neurology 2001;8 Suppl 5:109‐19. [PUBMED: 11851739] - PubMed
Brin 1997
-
- Brin MF. Botulinum toxin: chemistry, pharmacology, toxicity, and immunology. Muscle & Nerve. Supplement 1997;6:S146‐68. [PUBMED: 9826987] - PubMed
Brooks 1954
Cardoso 2006
Cohen 1988
-
- Cohen J. Statistical Power Analysis in the Behavioural Sciences. 2nd Edition. Hillsdale (NJ): Lawrence Erlbaum Associates Inc, 1988.
Copeland 2014a
-
- Copeland L, Edwards P, Thorley M, Donaghey S, Gascoigne‐Pees L, Kentish M, et al. Botulinum toxin A for nonambulatory children with cerebral palsy: a double blind randomized controlled trial. Journal of Pediatrics 2014;165(1):140‐6.e4. [DOI: 10.1016/j.jpeds.2014.01.050; PUBMED: 24630348] - DOI - PubMed
Delgado 2016a
Desloovere 2007
-
- Desloovere K, Molenaers G, Cat J, Pauwels P, Campenhout A, Ortibus E, et al. Motor function following multilevel botulinum toxin type A treatment in children with cerebral palsy. Developmental Medicine & Child Neurology 2007;49(1):56‐61. [DOI: 10.1017/s001216220700014x.x; PUBMED: 17209978] - DOI - PubMed
Druschel 2013
Edwards 2015
Feldman 1990
García Salazar 2015
-
- García Salazar LF, Dos Santos GL, Pavão SL, Rocha NA, Russo TL. Intrinsic properties and functional changes in spastic muscle after application of BTX‐A in children with cerebral palsy: systematic review. Developmental Neurorehabilitation 2015;18(1):1‐14. [DOI: 10.3109/17518423.2014.948640; PUBMED: 25180438] - DOI - PubMed
Gormley 2001
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro GDT: GRADEpro Guideline Development Tool. Version accessed 16 August 2019. Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.), 2015.
Graham 2008
-
- Graham HK, Boyd R, Carlin JB, Dobson F, Lowe K, Nattrass G, et al. Does botulinum toxin A combined with bracing prevent hip displacement in children with cerebral palsy and "hips at risk"? A randomized, controlled trial. Journal of Bone & Joint Surgery 2008;90(1):23‐33. [DOI: 10.2106/JBJS.F.01416; PUBMED: 18171954] - DOI - PubMed
Graham 2013
-
- Graham HK, Thomason P, Novacheck TF. Cerebral palsy. In: Weinstein SL, Flynn JM editor(s). Lovell & Winter's Pediatric Orthopaedics. 7th Edition. Philadelphia: Lippincott Williams & Wilkins, Wolters Kluwer Health, 2013:484‐554.
Gupta 2012
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC, editor(s), on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011a
-
- Higgins JPT, Deeks JJ, Altman DG, editor(s), on behalf of the Cochrane Statistical Methods Group. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Iyer 2003
-
- Iyer LV, Haley SM, Watkins MP, Dumas HM. Establishing minimal clinically important differences for scores on the Pediatric Evaluation of Disability Inventory for inpatient rehabilitation. Physical Therapy 2003;83(10):888‐98. [PUBMED: 14519060] - PubMed
Kiresuk 1968
Koman 1993
Koog 2010
Kostrzewa 2007
-
- Kostrzewa RM, Segura‐Aguilar J. Botulinum neurotoxin: evolution from poison, to research tool ‐ onto medicinal therapeutic and future pharmaceutical panacea. Neurotoxicity Research 2007;12(4):275‐90. [PUBMED: 18201955] - PubMed
Law 1990
Lee 2014
-
- Lee WY, Park GY, Kwon DR. Comparison of treatment effects between children with spastic cerebral palsy under and over five years after botulinum toxin type a injection. Annals of Rehabilitation Medicine 2014;38(2):200‐8. [DOI: 10.5535/arm.2014.38.2.200; PMC4026606; PUBMED: 24855614] - DOI - PMC - PubMed
Moher 2009
Molenaers 2013
Msall 1994
-
- Msall ME, DiGaudio K, Rogers BT, LaForest S, Catanzaro NL, Campbell J, et al. The Functional Independence Measure for Children (WeeFIM). Conceptual basis and pilot use in children with developmental disabilities. Clinical Pediatrics 1994;33(7):421‐30. [DOI: 10.1177/000992289403300708; PUBMED: 7525140] - DOI - PubMed
Naidu 2010
Narayanan 2006
-
- Narayanan UG, Fehlings D, Weir S, Knights S, Kiran S, Campbell K. Initial development and validation of the Caregiver Priorities and Child Health Index of Life with Disabilities (CPCHILD). Developmental Medicine & Child Neurology 2006;48(10):804‐12. - PubMed
Oeffinger 2008
-
- Oeffinger D, Bagley A, Rogers S, Gorton G, Kryscio R, Abel M, et al. Outcome tools used for ambulatory children with cerebral palsy: responsiveness and minimum clinically important differences. Developmental Medicine & Child Neurology 2008;50(12):918‐25. [DOI: 10.1111/j.1469-8749.2008.03150.x; PMC2990955; PUBMED: 19046185] - DOI - PMC - PubMed
Pascual‐Pascual 1997
-
- Pascual‐Pascual SI, Sánchez de Muniain P, Roche MC, Pascual‐Castroviejo I. Botulinum toxin as a treatment for infantile cerebral palsy. Revista de Neurologica 1997;25(145):1369‐75. [PUBMED: 9377292] - PubMed
Read 2003
-
- Read HS, Hazlewood ME, Hillman SJ, Prescott RJ, Robb JE. Edinburgh visual gait score for use in cerebral palsy. Journal of Pediatric Orthopaedics 2003;23(3):296‐301. [PUBMED: 12724590] - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rohatgi 2016 [Computer program]
-
- Rohatgi A. WebPlotDigitizer. Version 3.10. Austin (TX): Rohatgi A, 2016.
Rosenbaum 2007
-
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, et al. A report: the definition and classification of cerebral palsy April 2006. Developmental Medicine & Child Neurology. Supplement 2007;109:8‐14. [PUBMED: 17370477] - PubMed
RStudio 2018 [Computer program]
-
- RStudio, Inc. RStudio: Integrated Development for R.. Version 1.2.1335. Boston, MA: RStudio, Inc, 2018.
Ruiz 2004
Russell 1989
Ryll 2011
-
- Ryll U, Bastiaenen C, Bie R, Staal B. Effects of leg muscle botulinum toxin A injections on walking in children with spasticity‐related cerebral palsy: a systematic review. Developmental Medicine & Child Neurology 2011;53(3):210‐6. [DOI: 10.1111/j.1469-8749.2010.03890.x; PUBMED: 21291464] - DOI - PubMed
Sanger 2003
Scholtes 2006a
-
- Scholtes VA, Dallmeijer AJ, Knol DL, Speth LA, Maathuis CG, Jongerius PH, et al. The combined effect of lower‐limb multilevel botulinum toxin type A and comprehensive rehabilitation on mobility in children with cerebral palsy: a randomized clinical trial. Archives of Physical Medicine & Rehabilitation 2006;87(12):1551‐8. [DOI: 10.1016/j.apmr.2006.08.342; PUBMED: 17141633] - DOI - PubMed
Scholtes 2007
Schutte 2000
-
- Schutte LM, Narayanan U, Stout JL, Selber P, Gage JR, Schwartz MH. An index for quantifying deviations from normal gait. Gait & Posture 2000;11(1):25‐31. [PUBMED: 10664482] - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Schünemann 2017
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Akl E, et al. on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Scott 1980
Scott 1994
-
- Scott AB. Forward. In: Jankovic J, Hallet M editor(s). Therapy with Botulinum Toxin. New York (NY): Marcel Dekker Inc, 1994:vii‐ix.
Simpson 2008
-
- Simpson DM, Gracies JM, Graham HK, Miyasaki JM, Naumann M, Russman B, et al. Assessment: botulinum neurotoxin for the treatment of spasticity (an evidence‐based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2008;70(19):1691‐8. [PUBMED: 18458229] - PubMed
Sánchez‐Carpintero 1997
-
- Sánchez‐Carpintero R, Narbona J. Botulinum toxin in spastic infantile cerebral palsy: results in 27 cases during one year. Revista de Neurología 1997;25(140):531‐5. - PubMed
Tilton 2017
-
- Tilton A, Russman B, Aydin R, Dincer U, Escobar RG, Kutlay S, et al. AbobotulinumtoxinA (Dysport®) improves function according to goal attainment in children with dynamic equinus due to cerebral palsy. Journal of Child Neurology 2017;32(5):482‐7. [DOI: 10.1177/0883073816686910; PMC5405835; PUBMED: 28068857] - DOI - PMC - PubMed
Van der Houwen 2011
Waters 2007
WHO 2001
-
- World Health Organization. International Classification of Functioning, Disability and Health (ICF). Geneva: World Health Organization, 2001.
Wong 1998
Yang 1999
-
- Yang TF, Chan RC, Chuang TY, Liu TJ, Chiu JW. Treatment of cerebral palsy with botulinum toxin: evaluation with Gross Motor Function Measure. Journal of the Formosan Medical Association 1999;98(12):832‐6. [PUBMED: 10634023] - PubMed
Zmanovskaia 2014
-
- Zmanovskaia VA, Levitina EV, Popkov DA, Butorina MN, Pavlova OL. Botulinum toxin type A (Dysport) in the complex rehabilitation of children with spastic forms of cerebral palsy [Botulinicheskij toksin tipa A (disport) v kompleksnoj reabilitacii detej so spasticheskimi formami cerebral'nogo paralicha]. Zhurnal Nevrologii i Psikhiatrii Imeni SS Korsakova 2014;114(7):33‐6. [www.mediasphera.ru/issues/zhurnal‐nevrologii‐i‐psikhiatrii‐im‐s‐s‐korsak... - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

