Plastic Surgeons Defend Textured Breast Implants at 2019 U.S. Food and Drug Administration Hearing: Why It Is Time to Reconsider
- PMID: 31592028
- PMCID: PMC6756678
- DOI: 10.1097/GOX.0000000000002410
Plastic Surgeons Defend Textured Breast Implants at 2019 U.S. Food and Drug Administration Hearing: Why It Is Time to Reconsider
Abstract
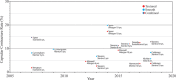
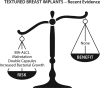
Textured breast implants were the subject of a U.S. Food and Drug Administration (FDA) hearing on March 25 and 26, 2019. Regulating agencies in other countries, including all of Europe and Canada, have already banned macrotextured implants. Patients affected by Breast Implant-Associated Anaplastic Large-Cell Lymphoma (BIA-ALCL) recounted their life-changing experiences, and requested a ban on textured devices. Plastic surgeons, many with industry ties, spoke in favor of keeping the devices available. The historical advantages of textured implants were presented, including a reduced capsular contracture rate. A 14-point plan to improve sterility at the time of implantation was promoted as an effective alternative to reduce both capsular contractures and BIA-ALCL risk. However, recent studies show that textured implants have not delivered on their early promise. Biocell implants perform worse, not better, than other implant types, and capsular contracture rates are not significantly reduced according to recent core studies. The only known risk factor for BIA-ALCL is textured implants. The lifetime risk for Biocell implants is at least 1:2, 200. There is no reliable evidence that surgical technique makes a difference in risk. This serious issue represents a case study of conflict of interest. In light of recent information, a re-analysis of the true risks and benefits of textured implants is justified. It is time for our professional societies to recognize that the device is the problem rather than surgical technique. On May 2, 2019, the FDA decided against a ban on textured breast implants.
Copyright © 2019 The Author. Published by Wolters Kluwer Health, Inc. on behalf of The American Society of Plastic Surgeons.
Figures

References
-
- 2019 Meeting Materials of the General and Plastic Surgery Devices Panel. https://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Medica.... Accessed April 7, 2019.
-
- Allergan Breast Implant Warranty Programme. https://www.cppwarranty.ca/assets/pdf/Canadian_CPP_Warranty_Terms_and_Co.... Accessed April 7, 2019.
-
- Webcast. General and Plastic Surgery Devices Panel Meeting. Day 1. http://fda.yorkcast.com/webcast/Play/a6baa43b37004ecab288779ac3a263bd1d. Accessed March 31, 2019.
-
- Webcast. General and Plastic Surgery Devices Meeting. Day 2. http://fda.yorkcast.com/webcast/Play/8f0ba1b148174747b4dbd0ec916167f11d. Accessed March 31, 2019.
-
- Adams WP, Jr., Culbertson EJ, Deva AK, et al. Macrotextured breast implants with defined steps to minimize bacterial contamination around the device: experience in 42,000 implants. Plast Reconstr Surg. 2017;140:427. - PubMed
LinkOut - more resources
Full Text Sources
