5-aminolaevulinic acid-based photodynamic therapy inhibits ultraviolet B-induced skin photodamage
- PMID: 31592145
- PMCID: PMC6775304
- DOI: 10.7150/ijbs.31583
5-aminolaevulinic acid-based photodynamic therapy inhibits ultraviolet B-induced skin photodamage
Abstract
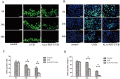
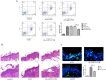
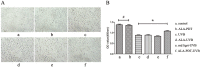
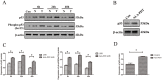
To evaluate the photoprotective effect of 5-aminolaevulinic acid-based photodynamic therapy (ALA-PDT) on ultraviolet B (UVB)-induced skin photodamage. In vivo experiments, the dorsal skin of hairless mice were treated with ALA-PDT or saline-PDT, and then exposed to 180 mJ/m2 UVB. Results showed that the number of sunburn cells and apoptotic cells in the epidermis of ALA-PDT-treated groups at 24 h after UVB irradiation were significantly decreased compared with those in the UVB groups. And the removal rate of CPDs was obviously higher in ALA-PDT-treated groups. At 48 h, the number of Ki67 positive nuclei in ALA-PDT-UVB group was significantly fewer than that in UVB group. Further in vitro experiments, human keratinocyte cell line (HaCaT) cells of two groups (one treated with ALA-PDT, the other untreated), were exposed to 60 mJ/m2 UVB irradiation. We found 0.5 mmol/L of ALA and 3 J/cm2 of red light did not affect the vitality of cells, and could reduce UVB induced apoptosis, accelerate the clearance of CPDs, inhibit proliferation and activate p53. Thus, our data demonstrate that ALA-PDT pretreatment can induce a protective DNA damage response that protects skin cells from UVB-induced photodamages.
Keywords: CPDs; DNA damage; p53; photodamage; photodynamic therapy; ultraviolet radiation.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
5-Aminolevulinic Acid-Based Photodynamic Therapy Pretreatment Mitigates Ultraviolet A-Induced Oxidative Photodamage.Oxid Med Cell Longev. 2018 Nov 7;2018:9420745. doi: 10.1155/2018/9420745. eCollection 2018. Oxid Med Cell Longev. 2018. Retraction in: Oxid Med Cell Longev. 2020 Aug 29;2020:2769472. doi: 10.1155/2020/2769472. PMID: 30524664 Free PMC article. Retracted.
-
ALA-PDT elicits oxidative damage and apoptosis in UVB-induced premature senescence of human skin fibroblasts.Photodiagnosis Photodyn Ther. 2016 Jun;14:47-56. doi: 10.1016/j.pdpdt.2016.02.005. Epub 2016 Feb 10. Photodiagnosis Photodyn Ther. 2016. PMID: 26876682
-
Systemic photodynamic therapy with aminolaevulinic acid delays the appearance of ultraviolet-induced skin tumours in mice.Br J Dermatol. 2001 Jun;144(6):1207-14. doi: 10.1046/j.1365-2133.2001.04232.x. Br J Dermatol. 2001. PMID: 11422043
-
Oxidative Stress and Photodynamic Therapy of Skin Cancers: Mechanisms, Challenges and Promising Developments.Antioxidants (Basel). 2020 May 22;9(5):448. doi: 10.3390/antiox9050448. Antioxidants (Basel). 2020. PMID: 32455998 Free PMC article. Review.
-
The influence of ultraviolet light on immunological cytotoxicity in the skin.Photochem Photobiol. 1997 Apr;65(4):636-46. doi: 10.1111/j.1751-1097.1997.tb01905.x. Photochem Photobiol. 1997. PMID: 9114739 Review. No abstract available.
Cited by
-
Reactive Oxygen Species Produced by 5-Aminolevulinic Acid Photodynamic Therapy in the Treatment of Cancer.Int J Mol Sci. 2023 May 18;24(10):8964. doi: 10.3390/ijms24108964. Int J Mol Sci. 2023. PMID: 37240309 Free PMC article. Review.
-
5-aminolevulinic acid photodynamic therapy protects against UVB-induced skin photoaging: A DNA-repairing mechanism involving the BER signalling pathway.J Cell Mol Med. 2024 Jul;28(14):e18536. doi: 10.1111/jcmm.18536. J Cell Mol Med. 2024. PMID: 39044341 Free PMC article.
References
-
- Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. The British journal of dermatology. 2012;166:1069–80. - PubMed
-
- Xiang F, Lucas R, Hales S, Neale R. Incidence of nonmelanoma skin cancer in relation to ambient UV radiation in white populations, 1978-2012: empirical relationships. JAMA dermatology. 2014;150:1063–71. - PubMed
-
- Seité S, Colige A, Piquemal-Vivenot P, Montastier C, Fourtanier A, Lapière C. et al. A full-UV spectrum absorbing daily use cream protects human skin against biological changes occurring in photoaging. Photodermatology Photoimmunology & Photomedicine. 2000;16:147–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

