Vascular Protection of TPE-CA on Hyperhomocysteinemia-induced Vascular Endothelial Dysfunction through AA Metabolism Modulated CYPs Pathway
- PMID: 31592228
- PMCID: PMC6775291
- DOI: 10.7150/ijbs.35245
Vascular Protection of TPE-CA on Hyperhomocysteinemia-induced Vascular Endothelial Dysfunction through AA Metabolism Modulated CYPs Pathway
Abstract
A high concentration of homocysteine (Hcy) in plasma induces vascular endothelial dysfunction, and it may ultimately accelerate the development of cardiovascular diseases (CVDs). Although several B vitamins have been clinically applied for hyperhomocysteinemia (HHcy) treatment, the outcomes are not satisfied due to their limited therapeutic mechanism. Hence, in order to improve the curative effect, development of new effective therapeutic strategies should be put on the agenda. Total phenolic extracts of Citrus aurantium L. (TPE-CA) is a naturally obtained phenolic mixture, mainly containing flavones, flavanones and their glycosyl derivatives, flavonols, polymethoxyflavones and coumarins. Previous reports indicated that bioactive phenolic compounds possessed potent vascular protective effects and regarded as a protective agent against CVDs. Intriguingly, the exact mechanism underlying the suppressed effects of TPE-CA on HHcy could assist in revealing their therapy on CVDs. Here, the multi-targeted synergistic mechanism of TPE-CA on HHcy-induced vascular endothelial dysfunction was uncovered in a deduced manner. TPE-CA treatment exhibited an obvious superiority than that of B vitamins treatment. Network pharmacology was employed to identify the interrelationships among compounds, potential targets and putative pathways. Further experimental validation suggested that the treatment of TPE-CA for HHcy could not only effectively reduce the Hcy level in plasma through up-regulating transsulfuration pathway in Hcy metabolism, but also restore the HHcy-induced vascular endothelial dysfunction by activating cytochrome P450 enzymes (CYPs) epoxygenase signal cascades and inhibiting CYPs hydroxylase signal cascades in arachidonic acid (AA) metabolism.
Keywords: Arachidonic acid metabolism; CYPs signal pathway; Hyperhomocysteinemia; Total phenolic extracts of Citrus aurantium L.; Vascular endothelial dysfunction.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
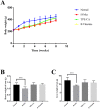







 Inhibition:
Inhibition:  Promotion by TPE-CA:
Promotion by TPE-CA:  Inhibition by TPE-CA:
Inhibition by TPE-CA: 
References
-
- Qaradakhi T, Matsoukas MT, Hayes A, Rybalka E, Caprnda M, Rimarova K, Alamandine reverses hyperhomocysteinemia-induced vascular dysfunction via PKA-dependent mechanisms. Cardiovascular therapeutics; 2017. p. 35. - PubMed
-
- Jamwal S, Sharma S. Vascular endothelium dysfunction: a conservative target in metabolic disorders. Inflammation research: official journal of the European Histamine Research Society [et al] 2018;67:391–405. - PubMed
-
- Chen JY, Ye ZX, Wang XF, Chang J, Yang MW, Zhong HH. et al. Nitric oxide bioavailability dysfunction involves in atherosclerosis. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2018;97:423–8. - PubMed
-
- Perez-Cremades D, Bueno-Beti C, Garcia-Gimenez JL, Ibanez-Cabellos JS, Hermenegildo C, Pallardo FV. et al. Extracellular histones disarrange vasoactive mediators release through a COX-NOS interaction in human endothelial cells. Journal of cellular and molecular medicine. 2017;21:1584–92. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

