Cluster-randomized crossover trial of chlorhexidine-alcohol versus iodine-alcohol for prevention of surgical-site infection (SKINFECT trial)
- PMID: 31592513
- PMCID: PMC6773639
- DOI: 10.1002/bjs5.50177
Cluster-randomized crossover trial of chlorhexidine-alcohol versus iodine-alcohol for prevention of surgical-site infection (SKINFECT trial)
Abstract
Background: Surgical-site infection (SSI) is a serious surgical complication that can be prevented by preoperative skin disinfection. In Western European countries, preoperative disinfection is commonly performed with either chlorhexidine or iodine in an alcohol-based solution. This study aimed to investigate whether there is superiority of chlorhexidine-alcohol over iodine-alcohol for preventing SSI.
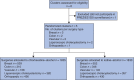
Methods: This prospective cluster-randomized crossover trial was conducted in five teaching hospitals. All patients who underwent breast, vascular, colorectal, gallbladder or orthopaedic surgery between July 2013 and June 2015 were included. SSI data were reported routinely to the Dutch National Nosocomial Surveillance Network (PREZIES). Participating hospitals were assigned randomly to perform preoperative skin disinfection using either chlorhexidine-alcohol (0·5 per cent/70 per cent) or iodine-alcohol (1 per cent/70 per cent) for the first 3 months of the study; every 3 months thereafter, they switched to using the other antiseptic agent, for a total of 2 years. The primary endpoint was the development of SSI.
Results: A total of 3665 patients were included; 1835 and 1830 of these patients received preoperative skin disinfection with chlorhexidine-alcohol or iodine-alcohol respectively. The overall incidence of SSI was 3·8 per cent among patients in the chlorhexidine-alcohol group and 4·0 per cent among those in the iodine-alcohol group (odds ratio 0·96, 95 per cent c.i. 0·69 to 1·35).
Conclusion: Preoperative skin disinfection with chlorhexidine-alcohol is similar to that for iodine-alcohol with respect to reducing the risk of developing an SSI.
Antecedentes: La infección del sitio quirúrgico (surgical site infection, SSI) es una complicación quirúrgica grave que se puede prevenir mediante una desinfección cutánea preoperatoria. En los países de Europa occidental, la desinfección preoperatoria se realiza habitualmente usando clorhexidina o yodo en una solución a base de alcohol. Nuestro objetivo fue investigar si la clorhexidina alcohólica es superior al yodo con alcohol para prevenir la SSI.
Métodos: Este ensayo prospectivo aleatorizado por conglomerados y de grupos cruzados se realizó en cinco hospitales docentes. Se incluyeron todos los pacientes que se sometieron a cirugía mamaria, vascular, colorrectal, biliar y ortopédica entre julio de 2013 y junio de 2015. Los datos de SSI se presentaron de manera rutinaria a la Red Nacional Holandesa de Vigilancia Nosocomial (PREZIES). Los hospitales participantes fueron asignados al azar para realizar una desinfección cutánea preoperatoria con clorhexidina alcohólica (0,5%/70%) o yodo con alcohol (1%/70%) durante los primeros tres meses del estudio; cada 3 meses a partir de entonces, cambiaron a usar el otro agente antiséptico, durante un total de 2 años. El criterio de valoración principal fue el desarrollo de SSI.
Resultados: Se incluyeron un total de 3.665 pacientes; 1.835 y 1.830 de estos pacientes recibieron desinfección cutánea preoperatoria con clorhexidina alcohólica o yodo con alcohol, respectivamente. La incidencia global de SSI fue del 3,8% entre los pacientes en el grupo de clorhexidina alcohólica y del 4,0% entre los pacientes en el grupo de yodo con alcohol (razón de oportunidades, odds ratio, OR 0,96; i.c. del 95%: 0,69‐1,35).
Conclusión: La desinfección cutánea preoperatoria con clorhexidina alcohólica es similar al yodo con alcohol con respecto a la reducción del riesgo de desarrollar una SSI.
© 2019 The Authors. BJS Open published by John Wiley & Sons Ltd on behalf of BJS Society Ltd.
Figures
Comment in
-
Cluster-randomized crossover trial of chlorhexidine-alcohol versus iodine-alcohol for prevention of surgical-site infection (SKINFECT trial).BJS Open. 2020 Aug;4(4):731-733. doi: 10.1002/bjs5.50285. Epub 2020 Apr 30. BJS Open. 2020. PMID: 32352222 Free PMC article. No abstract available.
References
-
- PREZIES (Dutch National Nosocomial Surveillance Network) . [Reference Numbers 2017 to 2014: Prevalence Research Hospitals] https://www.rivm.nl/sites/default/files/2018‐11/Referentiecijfers%20Prev... [accessed 7 May 2019].
-
- Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control 1992; 20: 271–274. - PubMed
-
- PREZIES . Protocol Module Postoperatieve Wondinfecties http://www.rivm.nl/Onderwerpen/P/PREZIES/Incidentieonderzoek_POWI/Protoc... [accessed 21 October 2017].
-
- Darouiche RO, Wall MJ Jr, Itani KM, Otterson MF, Webb AL, Carrick MM et al. Chlorhexidine–alcohol versus povidone–iodine for surgical‐site antisepsis. N Engl J Med 2010; 362: 18–26. - PubMed
-
- Charehbili A, van Gijn W, Liefers GJ, van de Velde C, Swijnenburg RJ. How evidence‐based is the transition from iodine to chlorhexidine for preoperative desinfection of the skin? Dutch J Surg 2013; 22: 34.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical