Blood Immune Cell Biomarkers in Patient With Lung Cancer Undergoing Treatment With Checkpoint Blockade
- PMID: 31592989
- PMCID: PMC7012348
- DOI: 10.1097/CJI.0000000000000297
Blood Immune Cell Biomarkers in Patient With Lung Cancer Undergoing Treatment With Checkpoint Blockade
Abstract
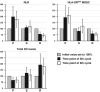
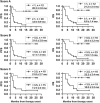
Characterization of host immune cell parameters before and during immunotherapy is expected to identify predictive biomarkers for clinical outcome. We prospectively monitored blood immune cells from 35 patients with advanced non-small cell lung cancer undergoing checkpoint inhibitor monotherapy. The aim was to identify parameters correlating with better/worse outcome. Peripheral blood was serially collected before each infusion at the onset and at cycle 3 and 5 of immunotherapy. A complete leukocyte blood count, the lymphocytic subpopulations and the percentages of both HLA-DR monocytes and dendritic cells (DC) were monitored. Disease control was defined as partial/complete response and stable disease on computed tomography scan according to RECIST 1.1. The predictive value of the immune cell parameters investigated was evaluated by patients' survival analysis. Forty percent of patients showed a clinical response, and the global median overall survival was 7.0 months (95% confidence interval: 3.5-10.5). Patients with an initial neutrophil-to-lymphocyte ratio (NLR) ≥5.2, and/or an amount of HLA-DR monocytes ≥11% and/or a total DC level ≤0.4% of leukocytes did rarely respond to PD-1 inhibitor therapy. Otherwise, the immunotherapy-induced decrease of the neutrophil-to-lymphocyte ratio and/or HLA-DR monocytes and the increase of total DC frequencies were correlated with improved therapy response and prolonged overall survival. Blood values in the third cycle of immunotherapy did already reflect the effects observed. On the basis of the 3 immune cell parameters identified we created 3 different variants of scores that enable to stratify patients into groups of risk/therapy response. Our results warrant further investigation in larger prospective clinical trials for validation.
Figures

References
-
- Scagliotti GV, Bironzo P, Vansteenkiste JF. Addressing the unmet need in lung cancer: the potential of immuno-oncology. Cancer Treat Rev. 2015;41:465–475. - PubMed
-
- Gandini S, Massi D, Mandala M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2016;100:88–98. - PubMed
-
- Rizvi H, Sanchez-Vega F, La K, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36:633–641. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

