Association of Surgical Hematoma Evacuation vs Conservative Treatment With Functional Outcome in Patients With Cerebellar Intracerebral Hemorrhage
- PMID: 31593272
- PMCID: PMC6784768
- DOI: 10.1001/jama.2019.13014
Association of Surgical Hematoma Evacuation vs Conservative Treatment With Functional Outcome in Patients With Cerebellar Intracerebral Hemorrhage
Abstract
Importance: The association of surgical hematoma evacuation with clinical outcomes in patients with cerebellar intracerebral hemorrhage (ICH) has not been established.
Objective: To determine the association of surgical hematoma evacuation with clinical outcomes in cerebellar ICH.
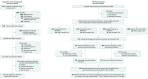
Design, setting, and participants: Individual participant data (IPD) meta-analysis of 4 observational ICH studies incorporating 6580 patients treated at 64 hospitals across the United States and Germany (2006-2015).
Exposure: Surgical hematoma evacuation vs conservative treatment.
Main outcomes and measures: The primary outcome was functional disability evaluated by the modified Rankin Scale ([mRS] score range: 0, no functional deficit to 6, death) at 3 months; favorable (mRS, 0-3) vs unfavorable (mRS, 4-6). Secondary outcomes included survival at 3 months and at 12 months. Analyses included propensity score matching and covariate adjustment, and predicted probabilities were used to identify treatment-related cutoff values for cerebellar ICH.
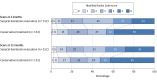
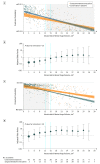
Results: Among 578 patients with cerebellar ICH, propensity score-matched groups included 152 patients with surgical hematoma evacuation vs 152 patients with conservative treatment (age, 68.9 vs 69.2 years; men, 55.9% vs 51.3%; prior anticoagulation, 60.5% vs 63.8%; and median ICH volume, 20.5 cm3 vs 18.8 cm3). After adjustment, surgical hematoma evacuation vs conservative treatment was not significantly associated with likelihood of better functional disability at 3 months (30.9% vs 35.5%; adjusted odds ratio [AOR], 0.94 [95% CI, 0.81 to 1.09], P = .43; adjusted risk difference [ARD], -3.7% [95% CI, -8.7% to 1.2%]) but was significantly associated with greater probability of survival at 3 months (78.3% vs 61.2%; AOR, 1.25 [95% CI, 1.07 to 1.45], P = .005; ARD, 18.5% [95% CI, 13.8% to 23.2%]) and at 12 months (71.7% vs 57.2%; AOR, 1.21 [95% CI, 1.03 to 1.42], P = .02; ARD, 17.0% [95% CI, 11.5% to 22.6%]). A volume range of 12 to 15 cm3 was identified; below this level, surgical hematoma evacuation was associated with lower likelihood of favorable functional outcome (volume ≤12 cm3, 30.6% vs 62.3% [P = .003]; ARD, -34.7% [-38.8% to -30.6%]; P value for interaction, .01), and above, it was associated with greater likelihood of survival (volume ≥15 cm3, 74.5% vs 45.1% [P < .001]; ARD, 28.2% [95% CI, 24.6% to 31.8%]; P value for interaction, .02).
Conclusions and relevance: Among patients with cerebellar ICH, surgical hematoma evacuation, compared with conservative treatment, was not associated with improved functional outcome. Given the null primary outcome, investigation is necessary to establish whether there are differing associations based on hematoma volume.
Conflict of interest statement
Figures

Comment in
-
Cerebellar Intracerebral Hemorrhage: Time for Evidence-Based Treatment.JAMA. 2019 Oct 8;322(14):1355-1356. doi: 10.1001/jama.2019.14673. JAMA. 2019. PMID: 31593256 No abstract available.
-
Unmeasured Confounding in Observational Studies of Management of Cerebellar Intracranial Hemorrhage.JAMA. 2020 Feb 18;323(7):665-666. doi: 10.1001/jama.2019.20851. JAMA. 2020. PMID: 32068811 No abstract available.
References
-
- Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al. ; Global Burden of Diseases, Injuries, Risk Factors Study 2010 (GBD 2010); GBD Stroke Experts Group . Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1(5):e259-e281. doi:10.1016/S2214-109X(13)70089-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

