Molecular Magnetic Resonance Imaging with Gd(III)-Based Contrast Agents: Challenges and Key Advances
- PMID: 31593630
- PMCID: PMC6821590
- DOI: 10.1021/jacs.9b09149
Molecular Magnetic Resonance Imaging with Gd(III)-Based Contrast Agents: Challenges and Key Advances
Abstract
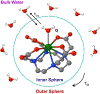
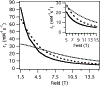
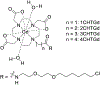
In an era of personalized medicine, the clinical community has become increasingly focused on understanding diseases at the cellular and molecular levels. Magnetic resonance imaging (MRI) is a powerful imaging modality for acquiring anatomical and functional information. However, it has limited applications in the field of molecular imaging due to its low sensitivity. To expand the capability of MRI to encompass molecular imaging applications, we introduced bioresponsive Gd(III)-based magnetic resonance contrast agents (GBCAs) in 1997. Since that time, many research groups across the globe have developed new examples of bioresponsive GBCAs. These contrast agents have shown great promise for visualizing several biochemical processes, such as gene expression, neuronal signaling, and hormone secretion. They are designed to be conditionally retained, or activated, in vivo in response to specific biochemical events of interest. As a result, an observed MR signal change can serve as a read-out for molecular events. A significant challenge for these probes is how to utilize them for noninvasive diagnostic and theranostic applications. This Perspective focuses on the design strategies that underlie bioresponsive probes, and describes the key advances made in recent years that are facilitating their application in vivo and ultimately in clinical translation. While the field of bioresponsive agents is embryonic, it is clear that many solutions to the experimental and clinical radiologic problems of today will be overcome by the probes of tomorrow.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




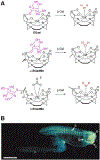
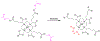
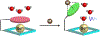







References
-
- Carr DH; Brown J; Bydder GM; Weinmann HJ; Speck U; Thomas DJ; Young IR Intravenous Chelated Gadolinium as a Contrast Agent in NMR Imaging of Cerebral-Tumors. Lancet 1984, 1, 484–486; - PubMed
- Helm L; Merbach AE; Tóth E. v.; ProQuest (Firm), The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging. 2nd ed.; John Wiley & Sons Inc.: Hoboken, N.J., 2013. http://ebookcentral.proquest.com/lib/northeastern-ebooks/detail.action?d....
-
- Weissleder R; ProQuest (Firm), Molecular Imaging Principles and Practice. People’s Medical Pub. House: Shelton, Conn., 2009. http://ebookcentral.proquest.com/lib/northeasternebooks/detail.action?do....
-
- Moats RA; Fraser SE; Meade TJA “Smart” Magnetic Resonance Imaging Agent That Reports on Specific Enzymatic Activity. Angew Chem Int Edit 1997, 36, 726–728;
- Heffern MC; Matosziuk LM; Meade TJ Lanthanide Probes for Bioresponsive Imaging. Chem. Rev 2014, 114, 4496–539; - PMC - PubMed
- Hingorani DV; Bernstein AS; Pagel MD A Review of Responsive MRI Contrast Agents: 2005–2014. Contrast Media Mol. Imaging 2015, 10, 245–265; - PMC - PubMed
- Lux J; Sherry AD Advances in Gadolinium-Based MRI Contrast Agent Designs for Monitoring Biological Processes in Vivo. Curr. Opin. Chem. Biol 2018, 45, 121–130; - PMC - PubMed
- Wahsner J; Gale EM; Rodriguez-Rodriguez A; Caravan P Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev 2019, 119, 957–1057; - PMC - PubMed
- Que EL; Chang CJ Responsive Magnetic Resonance Imaging Contrast Agents as Chemical Sensors for Metals in Biology and Medicine. Chem. Soc. Rev 2010, 39, 51–60. - PubMed
-
- Lu Y; Aimetti AA; Langer R; Gu Z Bioresponsive Materials. Nat Rev Mater 2017, 2.
-
- Prince MR; Zhang HL; Zou ZT; Staron RB; Brill PW Incidence of Immediate Gadolinium Contrast Media Reactions. Am. J. Roentgenol 2011, 196, W138–W143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

