The Role of Hypothalamic Pathology for Non-Motor Features of Huntington's Disease
- PMID: 31594240
- PMCID: PMC6839491
- DOI: 10.3233/JHD-190372
The Role of Hypothalamic Pathology for Non-Motor Features of Huntington's Disease
Abstract
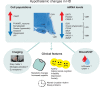
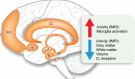
Huntington's disease (HD) is a fatal genetic neurodegenerative disorder. It has mainly been considered a movement disorder with cognitive symptoms and these features have been associated with pathology of the striatum and cerebral cortex. Importantly, individuals with the mutant huntingtin gene suffer from a spectrum of non-motor features often decades before the motor disorder manifests. These symptoms and signs include a range of psychiatric symptoms, sleep problems and metabolic changes with weight loss particularly in later stages. A higher body mass index at diagnosis is associated with slower disease progression. The common psychiatric symptom of apathy progresses with the disease. The fact that non-motor features are present early in the disease and that they show an association to disease progression suggest that unravelling the underlying neurobiological mechanisms may uncover novel targets for early disease intervention and better symptomatic treatment. The hypothalamus and the limbic system are important brain regions that regulate emotion, social cognition, sleep and metabolism. A number of studies using neuroimaging, postmortem human tissue and genetic manipulation in animal models of the disease has collectively shown that the hypothalamus and the limbic system are affected in HD. These findings include the loss of neuropeptide-expressing neurons such as orexin (hypocretin), oxytocin, vasopressin, somatostatin and VIP, and increased levels of SIRT1 in distinct nuclei of the hypothalamus. This review provides a summary of the results obtained so far and highlights the potential importance of these changes for the understanding of non-motor features in HD.
Keywords: Huntington’s disease; hypocretin; hypothalamus; orexin; oxytocin; vasopressin.
Conflict of interest statement
The authors have no conflict of interest to report.
Figures
References
-
- Ross CA, Aylward EH, Wild EJ, Langbehn DR, Long JD, Warner JH, et al. Huntington disease: Natural history, biomarkers and prospects for therapeutics. Nat Rev Neurol. 2014;10(4):204–16. - PubMed
-
- Duff K, Paulsen JS, Beglinger LJ, Langbehn DR, Stout JC, Predict-HD Investigators of the Huntington Study Group. Psychiatric symptoms in Huntington’s disease before diagnosis: The predict-HD study. Biol Psychiatry. 2007;62(12):1341–6. - PubMed
-
- Tabrizi SJ, Scahill RI, Durr A, Roos RA, Leavitt BR, Jones R, et al. Biological and clinical changes in premanifest and early stage Huntington’s disease in the TRACK-HD study: The 12-month longitudinal analysis. Lancet Neurol. 2011;10(1):31–42. - PubMed
-
- van Duijn E, Kingma EM, van der Mast RC. Psychopathology in verified Huntington’s disease gene carriers. J Neuropsychiatry Clin Neurosci. 2007;19(4):441–8. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

