Safety and Treatment Effects of Nusinersen in Longstanding Adult 5q-SMA Type 3 - A Prospective Observational Study
- PMID: 31594243
- PMCID: PMC6918909
- DOI: 10.3233/JND-190416
Safety and Treatment Effects of Nusinersen in Longstanding Adult 5q-SMA Type 3 - A Prospective Observational Study
Abstract
Objective: Spinal muscular atrophy (SMA) is a progressive autosomal recessive motor neuron disease caused by loss of the SMN1 gene. Based on randomized clinical trials in children with SMA type 1 and 2, Nusinersen has been approved as the first treatment for all types of SMA, including adults with SMA type 3.
Methods: We evaluated the safety and treatment effects of Nusinersen in longstanding adult 5q-SMA type 3. Patients were treated with intrathecal loading doses at day 1, 14, 28 and 63, followed by maintenance dose every four months up to 300 days. We monitored the patients within SMArtCARE, a prospective open-label outcome study for disease progression, side effects and treatment efficacy, encompassing clinical examination including MRC sum score, vital capacity in sitting position (VC, VC % pred.), ALS Functional Rating Scale (ALS-FRS), 6-Minute-Walk-Test (6MWT), Revised Upper Limb Module (RULM), and Hammersmith Functional Rating Scale (HFMSE). We also measured biomarkers in the spinal fluid (phosphorylated neurofilament heavy chain pNFH, neuron-specific enolase NSE, proteins, ß-Amyloid 1-40, ß-Amyloid 1-42, tau and phospho-tau) and creatine kinase (CK). Assessments were performed at baseline, day 63 (V4), day 180 (V5) and day 300 (V6). For statistical analysis, we compared baseline to V4, V5 and V6, using the paired sample t-test. When there were significant differences, we added cohen's d and effect size r for evaluation of clinical meaningfulness.
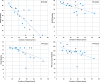
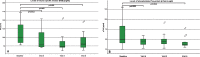
Results: 19 patients were included, 17 of them have completed the observation period of 10 months (day 300, V6). Patients were aged 18 to 59 years with disease duration ranging from 6 to 53 years. Except for the 6MWT, the RULM and the peak cough flow, there were no relevant significant changes in all functional outcome assessments at V4, V5 or V6, compared to baseline. For the 6MWT, there was a statistically significant improvement at visit 5 and at visit 6. RULM-score increased significantly at V6, and peak cough flow at visit 5. In biomarker studies, there was a significant decline in NSE and pTAU as well as a slight increase in proteins. In safety analysis, overall, Nusinersen applications were well tolerated. Eleven patients reported adverse events that were related to the study procedures, comprising back pain in seven patients and post-lumbar-puncture headache following intrathecal administration in four patients. Post-lumbar-puncture headache was reported in three females and one male, in total eleven times of 108 punctures (10%). No serious adverse events occurred.
Conclusions: This prospective observational study indicates a mild treatment effect in adults with long-standing SMA3 after 10 months of treatment with Nusinersen, which had never occurred in the natural history of the disease. In our cohort, the most significant outcome measures were the 6MWT with statistically significant changes after day 180 and day 300, RULM after day 300 and peak cough flow after day 180.
Keywords: Nusinersen; prospective observational study; safety; spinal muscular atrophy; treatment.
Conflict of interest statement
Simone Thiele, Julia Stauber, Miriam Hiebeler, Eva Greckl, Kristina Stahl report no disclosures.
Astrid Pechmann received funding from Biogen for educational activities.
Hanns Lochmüller: Consultancy and financial support for research projects and clinical trials from AMO Pharma, Biogen, Desitin, GW Pharma, Pfizer, PTC Therapeutics, Roche, Santhera, Sarepta, Satellos and Ultragenyx. Editor-in-chief for the Journal of Neuromuscular Disease (IOS Press)
Benedikt Schoser has served on advisory boards for Amicus Therapeutics, Audentes, Nexien, Lupin, Valerion, Vertex, and Sanofi Genzyme; he has undertaken contracted unrestricted research for Greenovation Biopharm, Sanofi Genzyme, Nexien; and has received honoraria from Octapharm and Kedrion, and travel expenses from Amicus and Sanofi Genzyme.
Janbernd Kirschner received funding from Avexis, Biogen, and Roche for advisory activities and clinical research activities related to spinal muscular atrophy.
Maggie C. Walter has served on advisory boards for Avexis, Biogen, Novartis, Roche, Santhera, Sarepta, PTC Therapeutics, Ultragenyx, Wave Sciences, received funding for Travel or Speaker Honoraria from Novartis, Biogen, Ultragenyx, Santhera, PTC Therapeutics, and worked as an ad-hoc consultant for AskBio, Audentes Therapeutics, Biogen Pharma GmbH, Fulcrum Therapeutics, GLG Consult, Guidepoint Global, Gruenenthal Pharma, Novartis, PTC Therapeutics.
Stephan Wenninger has served on advisory boards for Alexion Pharma, CSL Behring and Sanofi Genzyme GmbH. He received funding for Travel or Speaker Honoraria from Sanofi-Aventis Germany GmbH; SH Glykogenose Gesellschaft, Germany; AbbVie Germany GmbH; Recordati Pharma GmbH, Germany; CSL-Behring GmbH, Germany; Alexion Pharma Germany GmbH; Desitin Germany.
Figures


References
-
- Wadman RI, et al. Muscle strength and motor function throughout life in a cross-sectional cohort of 180 patients with spinal muscular atrophy types 1c-4. Eur J Neurol. 2018;25(3):512–8. - PubMed
-
- Finkel RS, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377(18):1723–32. - PubMed
-
- Mercuri E, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378(7):625–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

