One Year of Newborn Screening for SMA - Results of a German Pilot Project
- PMID: 31594245
- PMCID: PMC6918901
- DOI: 10.3233/JND-190428
One Year of Newborn Screening for SMA - Results of a German Pilot Project
Abstract
Objective: Spinal muscular atrophy (SMA) is the most common neurodegenerative disease in childhood. The study was conducted to assess the impact of early detection of SMA by newborn screening (NBS) on the clinical course of the disease.
Methods: Screening was performed in two federal states of Germany, Bavaria and North Rhine Westphalia, between January 2018 and February 2019. The incidence in the screening population was calculated as number of detected patients with a homozygous deletion in the SMN1-gene per number of screened patients. To get an idea about the incidence of newly diagnosed SMA in the year prior to screening a survey covering all neuropediatric centers in the state of Bavaria was conducted, identifying all SMA-cases in 2017 and 2018. Following positive NBS and confirmatory diagnostic test, treatment was advised according to the recommendations of the "American SMA NBS Multidisciplinary Working Group". Immediate treatment with Nusinersen was recommended in children with 2 and 3 SMN2 copies and a conservative strict follow-up strategy in children with ≥4 copies. All children underwent regular standardized neuropediatric examination, CHOP INTEND and HINE-2 testing as well as electrophysiological exams every 2-3 months.
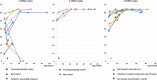
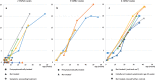
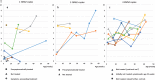
Results: 165,525 children were screened. 22 cases of SMA were identified, meaning an incidence rate of 1:7524. SMN2 copy number analysis showed 2 SMN2 copies in 45% of patients, 3 SMN2 copies in 19 % and 4 SMN2 copies in 36%. These findings are confirmed in the most recent statistical data-cut from 31st August 2019 (incidence 1:7089, 2 SMN2 copies in 44%, 3 in 15% and 4 in 38%). Comparison with up-to-date German data on SMA incidence and the Bavarian survey give evidence that NBS did not lead to a relevant increase in incidence. 10 patients with 2 or 3 SMN2 copies were treated with Nusinersen, starting between 15- 39 days after birth, in 7/10 patients before onset of symptoms. Presymptomatically treated patients (age at last examination: 1- 12 months, median 8 months) showed no muscle weakness by the age of one month to one year. One child with 4 SMN2 copies became symptomatic at the age of 8 months.
Conclusions: Newborn screening, resulting in presymptomatic treatment, improves outcome in children with genetically proven SMA. Newborn screening for SMA should be introduced in all countries where therapy is available. An immediate therapy in cases with 4 SMN2 copies should be considered.
Keywords: Nusinersen; SMA treatment; Spinal muscular atrophy; newborn screening; prognosis.
Conflict of interest statement
1. Serving on a scientific advisory board or data safety monitoring board.
2. Gifts (other than travel or compensation for consulting or for educational efforts) worth more than USD $1000.
3. Funding for travel or speaker honoraria to the individual from a commercial or non-profit entity not included in the study funding [Exclude CME activities and Grand Rounds].
4. Serving as a journal editor, an associate editor, or editorial advisory board member. This may include a journal published by your national medical/scientific organization. Please include regardless of whether you receive compensation.
5. Patents issued or pending.
6. Publishing Royalties (do not include honoraria for occasional writing).
7. Employment. If you are currently employed by a commercial entity, please disclose below. In addition, if your past employment at a commercial entity is directly related to this manuscript, please disclose below.
8. Consultancies.
9. Speakers’ bureau.
10. Other activities not covered in designations above (if in doubt, provide full disclosure).
11. Some published work has potential for financial gain for the study investigators or the sponsor. The following question seeks to provide transparency regarding any financial benefits to investigators or sponsors.
Katharina Vill, Heike Kölbel, Oliver Schwartz, Astrid Blaschek, Bernhard Olgemöller, Erik Harms, Uta Nennstiel, and Beate Jensen have nothing to declare.
Siegfried Burggraf, Wulf Röschinger, Jürgen Durner and Marc Becker are employed by/owner of a commercial entity (Laboratory Becker and colleagues MVZ GbR, Führichstraße 70, 81871 München, Germany).
Dieter Gläser: employed by/owner of a commercial entity (Genetikum®, Wegenerstr. 15, 89231 Neu-Ulm, Germany).
Ulrika Schara is serving on a scientific advisory board or data safety monitoring board for Biogen, Avexis and Novartis.
Brunhilde Wirth is serving on a scientific advisory board or data safety monitoring board for SMA Europe and received travel and speaker honoraria from Biogen.
Katharina Hohenfellner received commercial travel support and speaker honoraria from Ortphan Europe, Chiesi, and non-profit travel support from Cystinosis Foundation and Nephie.
Wolfgang Müller-Felber is serving on a scientific advisory board or data safety monitoring board for Biogen, Avexis, PTC, Sanofi-Aventis and Cytokinetics and received travel and speaker honoraria from Biogen, Avexis, PTC and Sanofi-Aventis.
Figures
References
-
- Wang CH, Finkel RS, Bertini ES, Schroth M, Simonds A, Wong B, et al. Consensus statement for standard of care in spinal muscular atrophy. Journal of Child Neurology. 2007;22(8):1027–49. - PubMed
-
- Mercuri E, Finkel RS, Muntoni F, Wirth B, Montes J, Main M, et al. Diagnosis and management of spinal muscular atrophy: Part Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscular Disorders: NMD. 2018;28(2):103–15. - PubMed
-
- Finkel RS, Mercuri E, Meyer OH, Simonds AK, Schroth MK, Graham RJ, et al. Diagnosis and management of spinal muscular atrophy: Part 2: Pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscular disorders: NMD. 2018;28(3):197–207. - PubMed
-
- Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, Viollet L, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80(1):155–65. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

