Evaluating phase separation in live cells: diagnosis, caveats, and functional consequences
- PMID: 31594803
- PMCID: PMC6942051
- DOI: 10.1101/gad.331520.119
Evaluating phase separation in live cells: diagnosis, caveats, and functional consequences
Abstract
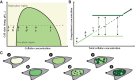
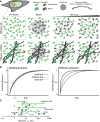
The idea that liquid-liquid phase separation (LLPS) may be a general mechanism by which molecules in the complex cellular milieu may self-organize has generated much excitement and fervor in the cell biology community. While this concept is not new, its rise to preeminence has resulted in renewed interest in the mechanisms that shape and drive diverse cellular self-assembly processes from gene expression to cell division to stress responses. In vitro biochemical data have been instrumental in deriving some of the fundamental principles and molecular grammar by which biological molecules may phase separate, and the molecular basis of these interactions. Definitive evidence is lacking as to whether the same principles apply in the physiological environment inside living cells. In this Perspective, we analyze the evidence supporting phase separation in vivo across multiple cellular processes. We find that the evidence for in vivo LLPS is often phenomenological and inadequate to discriminate between phase separation and other possible mechanisms. Moreover, the causal relationship and functional consequences of LLPS in vivo are even more elusive. We underscore the importance of performing quantitative measurements on proteins in their endogenous state and physiological abundance, as well as make recommendations for experiments that may yield more conclusive results.
Keywords: condensate; fluorescence recovery after photobleaching; liquid–liquid phase separation; phase separation.
© 2019 McSwiggen et al.; Published by Cold Spring Harbor Laboratory Press.
Figures