Fitness effects but no temperature-mediated balancing selection at the polymorphic Adh gene of Drosophila melanogaster
- PMID: 31594844
- PMCID: PMC6815130
- DOI: 10.1073/pnas.1909216116
Fitness effects but no temperature-mediated balancing selection at the polymorphic Adh gene of Drosophila melanogaster
Abstract
Polymorphism in the alcohol dehydrogenase (ADH) protein of Drosophila melanogaster, like genetic variation in many other enzymes, has long been hypothesized to be maintained by a selective trade-off between thermostability and enzyme activity. Two major Adh variants, named Fast and Slow, are distributed along latitudinal clines on several continents. The balancing selection trade-off hypothesis posits that Fast is favored at high latitudes because it metabolizes alcohol faster, whereas Slow is favored at low latitudes because it is more stable at high temperatures. Here we use biochemical and physiological assays of precisely engineered genetic variants to directly test this hypothesis. As predicted, the Fast protein has higher catalytic activity than Slow, and both the Fast protein and regulatory variants linked to it confer greater ethanol tolerance on transgenic animals. But we found no evidence of a temperature-mediated trade-off: The Fast protein is not less stable or active at high temperatures, and Fast alleles increase ethanol tolerance and survivorship at all temperatures tested. Further, analysis of a population genomic dataset reveals no signature of balancing selection in the Adh gene. These results provide strong evidence against balancing selection driven by a stability/activity trade-off in Adh, and they justify caution about this hypothesis for other enzymes except those for which it has been directly tested. Our findings tentatively suggest that environment-specific selection for the Fast allele, coupled with demographic history, may have produced the observed pattern of Adh variation.
Keywords: activity/stability trade-off; adaptation; alcohol physiology; evolutionary biochemistry; evolutionary genetics.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


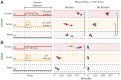

References
-
- Lewontin R. C., The Genetic Basis of Evolutionary Change (Columbia University Press, New York, NY, 1974).
-
- Gillespie J. H., The Causes of Molecular Evolution (Oxford University Press, 1994).
-
- Vitti J. J., Grossman S. R., Sabeti P. C., Detecting natural selection in genomic data. Annu. Rev. Genet. 47, 97–120 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

