Randomized trial of daily high-dose vitamin D3 in patients with RRMS receiving subcutaneous interferon β-1a
- PMID: 31594857
- PMCID: PMC6946471
- DOI: 10.1212/WNL.0000000000008445
Randomized trial of daily high-dose vitamin D3 in patients with RRMS receiving subcutaneous interferon β-1a
Abstract
Objective: In the phase II, randomized, double-blind, placebo-controlled Supplementation of Vigantol Oil versus Placebo Add-on in Patients with Relapsing-Remitting Multiple Sclerosis (RRMS) Receiving Rebif Treatment (SOLAR) study (NCT01285401), we assessed the efficacy and safety of add-on vitamin D3 in patients with RRMS.
Methods: Eligible patients with RRMS treated with SC interferon-β-1a (IFN-β-1a) 44 μg 3 times weekly and serum 25(OH)D levels <150 nmol/L were included. From February 15, 2011, to May 11, 2015, 229 patients were included and randomized 1:1 to receive SC IFN-β-1a plus placebo (n = 116) or SC IFN-β-1a plus oral high-dose vitamin D3 14,007 IU/d (n = 113). The revised primary outcome was the proportion of patients with no evidence of disease activity (NEDA-3) at week 48.
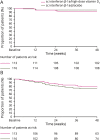
Results: At 48 weeks, 36.3% of patients who received high-dose vitamin D3 had NEDA-3, without a statistically significant difference in NEDA-3 status between groups (placebo 35.3%; odds ratio 0.93; 95% confidence interval [CI] 0.53-1.63; p = 0.80). Compared with placebo, the high-dose vitamin D3 group had better MRI outcomes for combined unique active lesions (incidence rate ratio 0.68; 95% CI 0.52-0.89; p = 0.0045) and change from baseline in total volume of T2 lesions (difference in mean ranks: -0.074; p = 0.035).
Conclusions: SOLAR did not establish a benefit for high-dose vitamin D3 as add-on to IFN-β-1a, based on the primary outcome of NEDA-3, but findings from exploratory outcomes suggest protective effects on development of new MRI lesions in patients with RRMS.
Clinicaltrialsgov identifier: NCT01285401.
Classification of evidence: This study provides Class II evidence that for patients with RRMS treated with SC IFN-β-1a, 48 weeks of cholecalciferol supplementation did not promote NEDA-3 status.
Copyright © 2019 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
References
-
- Fitzgerald KC, Munger KL, Kochert K, et al. . Association of vitamin D levels with multiple sclerosis activity and progression in patients receiving interferon beta-1b. JAMA Neurol 2015;72:1458–1465. - PubMed
-
- Goldberg P, Fleming MC, Picard EH. Multiple sclerosis: decreased relapse rate through dietary supplementation with calcium, magnesium and vitamin D. Med Hypotheses 1986;21:193–200. - PubMed
-
- Polman CH, Reingold SC, Edan G, et al. . Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.” Ann Neurol 2005;58:840–846. - PubMed
-
- Correale J, Ysrraelit MC, Gaitan MI. Vitamin D-mediated immune regulation in multiple sclerosis. J Neurol Sci 2011;311:23–31. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
