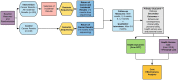
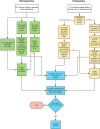
Health outcomes, utility and costs of returning incidental results from genomic sequencing in a Canadian cancer population: protocol for a mixed-methods randomised controlled trial
- PMID: 31594892
- PMCID: PMC6797333
- DOI: 10.1136/bmjopen-2019-031092
Health outcomes, utility and costs of returning incidental results from genomic sequencing in a Canadian cancer population: protocol for a mixed-methods randomised controlled trial
Abstract
Introduction: Genomic sequencing has rapidly transitioned into clinical practice, improving diagnosis and treatment options for patients with hereditary disorders. However, large-scale implementation of genomic sequencing faces challenges, especially with regard to the return of incidental results, which refer to genetic variants uncovered during testing that are unrelated to the primary disease under investigation, but of potential clinical significance. High-quality evidence evaluating health outcomes and costs of receiving incidental results is critical for the adoption of genomic sequencing into clinical care and to understand the unintended consequences of adoption of genomic sequencing. We aim to evaluate the health outcomes and costs of receiving incidental results for patients undergoing genomic sequencing.
Methods and analysis: We will compare health outcomes and costs of receiving, versus not receiving, incidental results for adult patients with cancer undergoing genomic sequencing in a mixed-methods randomised controlled trial. Two hundred and sixty patients who have previously undergone first or second-tier genetic testing for cancer and received uninformative results will be recruited from familial cancer clinics in Toronto, Ontario. Participants in both arms will receive cancer-related results. Participants in the intervention arm have the option to receive incidental results. Our primary outcome is psychological distress at 2 weeks following return of results. Secondary outcomes include behavioural consequences, clinical and personal utility assessed over the 12 months after results are returned and health service use and costs at 12 months and 5 years. A subset of participants and providers will complete qualitative interviews about utility of incidental results.
Ethics and dissemination: This study has been approved by Clinical Trials Ontario Streamlined Research Ethics Review System that provides ethical review and oversight for multiple sites participating in the same clinical trial in Ontario.Results from the trial will be shared through stakeholder workshops, national and international conferences, and peer-reviewed journals.
Trial registration number: NCT03597165.
Keywords: clinical utility; genomic sequencing; health care services use and costs; incidental findings; randomized controlled trial; secondary findings.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- National Human Genome Research Institute The cost of sequencing a human genome; 2016. https://www.genome.gov/about-genomics/fact-sheets/Sequencing-Human-Genom...
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical