Phytochrome evolution in 3D: deletion, duplication, and diversification
- PMID: 31595505
- PMCID: PMC7028483
- DOI: 10.1111/nph.16240
Phytochrome evolution in 3D: deletion, duplication, and diversification
Abstract
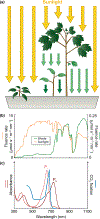
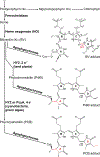
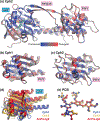
Canonical plant phytochromes are master regulators of photomorphogenesis and the shade avoidance response. They are also part of a widespread superfamily of photoreceptors with diverse spectral and biochemical properties. Plant phytochromes belong to a clade including other phytochromes from glaucophyte, prasinophyte, and streptophyte algae (all members of the Archaeplastida) and those from cryptophyte algae. This is consistent with recent analyses supporting the existence of an AC (Archaeplastida + Cryptista) clade. AC phytochromes have been proposed to arise from ancestral cyanobacterial genes via endosymbiotic gene transfer (EGT), but most recent studies instead support multiple horizontal gene transfer (HGT) events to generate extant eukaryotic phytochromes. In principle, this scenario would be compared to the emerging understanding of early events in eukaryotic evolution to generate a coherent picture. Unfortunately, there is currently a major discrepancy between the evolution of phytochromes and the evolution of eukaryotes; phytochrome evolution is thus not a solved problem. We therefore examine phytochrome evolution in a broader context. Within this context, we can identify three important themes in phytochrome evolution: deletion, duplication, and diversification. These themes drive phytochrome evolution as organisms evolve in response to environmental challenges.
Keywords: endosymbiosis; evolution; light harvesting; photosynthesis; shade avoidance.
© 2019 The Authors. New Phytologist © 2019 New Phytologist Trust.
Figures



References
-
- Armbrust EV, Berges JA, Bowler C, Green BR, Martinez D, Putnam NH, Zhou S, Allen AE, Apt KE, Bechner M, et al. 2004. The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 306(5693): 79–86. - PubMed
-
- Auldridge ME, Forest KT. 2011. Bacterial phytochromes: more than meets the light. Critical Reviews in Biochemistry and Molecular Biology. 46(1): 67–88. - PubMed
-
- Ballaré CL, Pierik R. 2017. The shade-avoidance syndrome: multiple signals and ecological consequences. Plant Cell and Environment 40(11): 2530–2543. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

