Convergent Total Syntheses of (-)-Rubriflordilactone B and (-)-pseudo-Rubriflordilactone B
- PMID: 31595605
- PMCID: PMC6973266
- DOI: 10.1002/anie.201908917
Convergent Total Syntheses of (-)-Rubriflordilactone B and (-)-pseudo-Rubriflordilactone B
Abstract
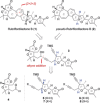
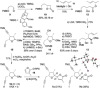
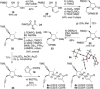
A highly convergent strategy for the synthesis of the natural product (-)-rubriflordilactone B, and the proposed structure of (-)-pseudo-rubriflordilactone B, is described. Late stage coupling of diynes containing the respective natural product FG rings with a common AB ring aldehyde precedes rhodium-catalyzed [2+2+2] alkyne cyclotrimerization to form the natural product skeleton, with the syntheses completed in just one further operation. This work resolves the uncertainty surrounding the identity of pseudo-rubriflordilactone B and provides a robust platform for further synthetic and biological investigations.
Keywords: cyclotrimerization; natural products; schinortriterpenoids; structure elucidation; total synthesis.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Total Synthesis of (+)-Rubriflordilactone A.Angew Chem Int Ed Engl. 2015 Oct 19;54(43):12618-21. doi: 10.1002/anie.201506366. Epub 2015 Sep 4. Angew Chem Int Ed Engl. 2015. PMID: 26337920 Free PMC article.
-
Total Synthesis of the Schisandraceae Nortriterpenoid Rubriflordilactone A.Chemistry. 2017 Oct 9;23(56):14080-14089. doi: 10.1002/chem.201703229. Epub 2017 Sep 8. Chemistry. 2017. PMID: 28768051 Free PMC article.
-
Enantioselective Total Synthesis of (-)-Rubriflordilactone B by a Bioinspired Skeletal Reorganization Approach.J Am Chem Soc. 2025 Mar 5;147(9):7875-7885. doi: 10.1021/jacs.4c18292. Epub 2025 Feb 17. J Am Chem Soc. 2025. PMID: 39960028
-
Synthetic Study of Rubriflordilactone B: Highly Stereoselective Construction of the C-5-epi ABCDE Ring System.Org Lett. 2016 Feb 19;18(4):792-5. doi: 10.1021/acs.orglett.6b00057. Epub 2016 Feb 2. Org Lett. 2016. PMID: 26836947
-
Interception of a Rautenstrauch intermediate by alkynes for [5+2] cycloaddition: rhodium-catalyzed cycloisomerization of 3-acyloxy-4-ene-1,9-diynes to bicyclo[5.3.0]decatrienes.Angew Chem Int Ed Engl. 2011 Aug 22;50(35):8153-6. doi: 10.1002/anie.201103136. Epub 2011 Jul 11. Angew Chem Int Ed Engl. 2011. PMID: 21748834 Free PMC article.
Cited by
-
Synthetic Study toward Triterpenes from the Schisandraceae Family of Natural Products.Molecules. 2023 May 31;28(11):4468. doi: 10.3390/molecules28114468. Molecules. 2023. PMID: 37298943 Free PMC article.
-
Enantioselective Total Synthesis of (+)-Heilonine.J Am Chem Soc. 2021 Oct 13;143(40):16394-16400. doi: 10.1021/jacs.1c08756. Epub 2021 Sep 29. J Am Chem Soc. 2021. PMID: 34585920 Free PMC article.
-
Streamlined Catalytic Enantioselective Synthesis of α-Substituted β,γ-Unsaturated Ketones and Either of the Corresponding Tertiary Homoallylic Alcohol Diastereomers.J Am Chem Soc. 2020 Oct 21;142(42):18200-18212. doi: 10.1021/jacs.0c08732. Epub 2020 Oct 5. J Am Chem Soc. 2020. PMID: 33016068 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- EP/L015838/1/Engineering and Physical Sciences Research Council/International
- EP/M019195/1/Engineering and Physical Sciences Research Council/International
- EP/E055273/1/Engineering and Physical Sciences Research Council/International
- GA No. 702385/H2020 Marie Skłodowska-Curie Actions/International
- Office of the Provost, Christopher Newport University/International
LinkOut - more resources
Full Text Sources

