B cell reconstitution following alemtuzumab induction under a belatacept-based maintenance regimen
- PMID: 31596034
- PMCID: PMC7202689
- DOI: 10.1111/ajt.15639
B cell reconstitution following alemtuzumab induction under a belatacept-based maintenance regimen
Abstract
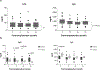
Lymphocyte depletion has been shown to control costimulation blockade-resistant rejection but, in some settings, to exacerbate antibody-mediated rejection (AMR). We have used alemtuzumab, which depletes T and B cells, combined with belatacept and rapamycin and previously reported control of both costimulation blockade-resistant rejection and AMR. To evaluate this regimen's effect on B cell signatures, we investigated 40 patients undergoing this therapy. B cell counts and phenotypes were interrogated using flow cytometry, and serum was analyzed for total IgG, IgM, and donor-specific alloantibody (DSA). Alemtuzumab induction produced pan-lymphocyte depletion; B cells repopulated faster and more completely than T cells. Reconstituting B cells were predominantly naïve, and memory B cells were significantly reduced (P = .001) post repopulation. Two B cell populations with potential immunomodulatory effects-regulatory (CD38hi CD24hi IgMhi CD20hi ) and transitional B cells (CD19+ CD27- IgD+ CD38hi )-were enriched posttransplant (P = .001). Total serum IgG decreased from baseline (P = .016) while IgM levels remained stable. Five patients developed DSAs within 36 months posttransplant, but none developed AMR. Baseline IgG levels in these patients were significantly higher than those in patients without DSAs. These findings suggest that belatacept and rapamycin together limit homeostatic B cell activation following B cell depletion and may lessen the risk of AMR. This regimen warrants prospective, comparative study. ClinicalTrials.gov NCT00565773.
Keywords: B cell biology; basic (laboratory) research/science; clinical research/practice; costimulation; immunosuppression/immune modulation; immunosuppressive regimens - induction; lymphocyte biology: differentiation/maturation.
© 2019 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
Figures


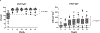
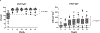
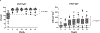

References
-
- Vicenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005; 353:770–781. - PubMed
-
- Vicenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L, et al. Belatacept and long-term outcomes in kidney transplantation. N Engl J Med. 2016; 374:333–343. - PubMed
-
- Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003; 349:931–940. - PubMed
-
- Fisher R. Cytomegalovirus infection and disease in the new era of immunosuppression following solid organ transplantation. Transpl Infect Dis. 2009; 11:199–225. - PubMed
-
- Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Dario P, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant 2010; 10:535–546. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

