High-Spin Diradical Dication of Chiral π-Conjugated Double Helical Molecule
- PMID: 31596077
- PMCID: PMC7039282
- DOI: 10.1021/jacs.9b08711
High-Spin Diradical Dication of Chiral π-Conjugated Double Helical Molecule
Abstract
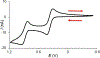
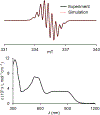
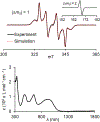
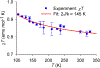
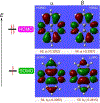
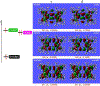
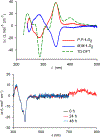
We report an air-stable diradical dication of chiral D2-symmetric conjoined bis[5]diazahelicene with an unprecedented high-spin (triplet) ground state, singlet triplet energy gap, ΔEST = 0.3 kcal mol-1. The diradical dication possesses closed-shell (Kekulé) resonance forms with 16 π-electron perimeters. The diradical dication is monomeric in dibutyl phthalate (DBP) matrix at low temperatures, and it has a half-life of more than 2 weeks at ambient conditions in the presence of excess oxidant. A barrier of ∼35 kcal mol-1 has been experimentally determined for inversion of configuration in the neutral conjoined bis[5]diazahelicene, while the inversion barriers in its radical cation and diradical dication were predicted by the DFT computations to be within a few kcal mol-1 of that in the neutral species. Chiral HPLC resolution provides the chiral D2-symmetric conjoined bis[5]diazahelicene, enriched in (P,P)- or (M,M)-enantiomers. The enantiomerically enriched triplet diradical dication is configurationally stable for 48 h at room temperature, thus providing the lower limit for inversion barrier of configuration of 27 kcal mol-1. The enantiomers of conjoined bis[5]diazahelicene and its diradical dication show strong chirooptical properties that are comparable to [6]helicene or carbon-sulfur [7]helicene, as determined by the anisotropy factors, |g| = |Δε|/ε = 0.007 at 348 nm (neutral) and |g| = 0.005 at 385 nm (diradical dication). DFT computations of the radical cation suggest that SOMO and HOMO energy levels are near-degenerate.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

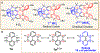

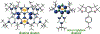
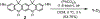
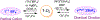
References
-
- Shen Y; Chen C-F Helicenes: synthesis and applications. Chem. Rev 2012, 112, 1463–1535. - PubMed
- Rajca A; Miyasaka M Synthesis and Characterization of Novel Chiral Conjugated Materials In Functional Organic Materials - Syntheses and Strategies; Mueller TJJ; Bunz UHF, Eds.; Wiley-VCH: New York, 2007; pp 543–577.
-
- Naaman R; Paltiel Y; Waldeck DH Chiral molecules and the electron spin. Nat. Rev. Chem 2019, 3, 250–260.
- Kira V; Mathew SP; Cohen SR; Delgado IH; Lacour J; Naaman R Helicenes—A New Class of Organic Spin Filter. Adv. Mater 2016, 28, 1957–1962. - PubMed
-
- Miyamoto Y; Rubio A; Cohen ML Self-inductance of chiral conducting nanotubes. Phys. Rev. B 1999, 60, 13885–13889.
- Rikken GLJA; Folling J; Wyder P Electrical Magnetochiral Anisotropy. Phys. Rev. Lett 2001, 87, 236602–1–4. - PubMed
- Sessoli R; Boulon M-E; Caneschi A; Mannini M; Poggini L; Wilhelm F; Rogalev A Strong magneto-chiral dichroism in a paramagnetic molecular helix observed by hard X-ray. Nat Phys. 2015, 11, 69–74. - PMC - PubMed
- Xu X; Li W; Zhou X; Wang Q; Feng J; Tian WQ; Jiang Y Theoretical study of electron tunneling through the spiral molecule junctions along spiral paths. Phys. Chem. Chem. Phys 2016, 18, 3765–3771. - PubMed
-
- Herrmann C; Solomon GC; Ratner MA Organic Radicals As Spin Filters. J. Am. Chem. Soc 2010, 132, 3682–3684. - PubMed
-
- Shil S; Bhattacharya D; Misra A; Klein DJ A high-spin organic diradical as a spin filter. Phys. Chem. Chem. Phys 2015, 17, 23378–23383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

